|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001760 |
|---|
|
Identification |
|---|
| Name: |
undecaprenyl phosphate-4-amino-4-deoxy-L-arabinose |
|---|
| Description: | The modified arabinose is attached to lipid A and is required for resistance to polymyxin and cationic antimicrobial peptides |
|---|
|
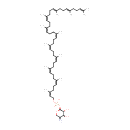
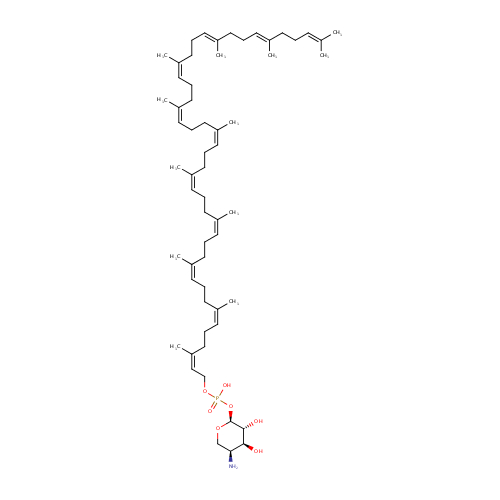
Structure |
|
|---|
| Synonyms: | - Undecaprenyl phosphoric acid-4-amino-4-deoxy-L-arabinose
- Undecaprenyl-P-Ara4N
|
|---|
|
Chemical Formula: |
C60H100NO7P |
|---|
| Average Molecular Weight: |
978.4123 |
|---|
| Monoisotopic Molecular
Weight: |
977.723741071 |
|---|
| InChI Key: |
BAFPKKRTAQMYMS-MEKAZKDWSA-N |
|---|
| InChI: | InChI=1S/C60H100NO7P/c1-46(2)23-13-24-47(3)25-14-26-48(4)27-15-28-49(5)29-16-30-50(6)31-17-32-51(7)33-18-34-52(8)35-19-36-53(9)37-20-38-54(10)39-21-40-55(11)41-22-42-56(12)43-44-67-69(64,65)68-60-59(63)58(62)57(61)45-66-60/h23,25,27,29,31,33,35,37,39,41,43,57-60,62-63H,13-22,24,26,28,30,32,34,36,38,40,42,44-45,61H2,1-12H3,(H,64,65)/b47-25+,48-27+,49-29-,50-31-,51-33-,52-35-,53-37-,54-39-,55-41-,56-43-/t57-,58-,59+,60-/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | {[(2S,3R,4S,5S)-5-amino-3,4-dihydroxyoxan-2-yl]oxy}({[(2Z,6Z,10Z,14Z,18Z,22Z,26Z,30Z,34E,38E)-3,7,11,15,19,23,27,31,35,39,43-undecamethyltetratetraconta-2,6,10,14,18,22,26,30,34,38,42-undecaen-1-yl]oxy})phosphinic acid |
|---|
|
Traditional IUPAC Name: |
[(2S,3R,4S,5S)-5-amino-3,4-dihydroxyoxan-2-yl]oxy[(2Z,6Z,10Z,14Z,18Z,22Z,26Z,30Z,34E,38E)-3,7,11,15,19,23,27,31,35,39,43-undecamethyltetratetraconta-2,6,10,14,18,22,26,30,34,38,42-undecaen-1-yl]oxyphosphinic acid |
|---|
| SMILES: | N[C@H]1CO[C@@H](OP(=O)(O)OC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(\C)CC\C=C(/C)CC\C=C(/C)CCC=C(C)C)[C@H](O)[C@H]1O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as bactoprenol monophosphates. These are polyprenyl compounds consisting of a monophosphate group substituted by a bactoprenyl moiety. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Prenol lipids |
|---|
| Sub Class | Polyprenols |
|---|
|
Direct Parent |
Bactoprenol monophosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Polyterpenoid
- Bactoprenol monophosphate
- Amino sugar
- Isoprenoid phosphate
- Dialkyl phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Oxane
- Organic phosphoric acid derivative
- Organic phosphate
- Monosaccharide
- Saccharide
- Secondary alcohol
- 1,2-diol
- 1,2-aminoalcohol
- Oxacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Amine
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Membrane |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Cationic antimicrobial peptide (CAMP) resistance pae01503
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | 60463 | | HMDB ID | Not Available | | Pubchem Compound ID | 44229076 | | Kegg ID | C16157 | | ChemSpider ID | 26331957 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|