|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001667 |
|---|
|
Identification |
|---|
| Name: |
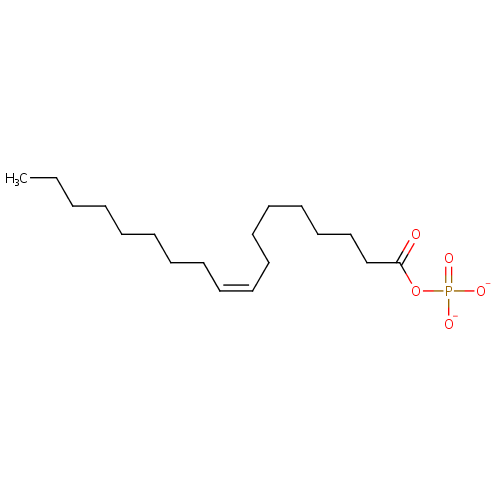
Octadecanoyl-phosphate (n-C18:1) |
|---|
| Description: | Caprylic acid belongs to the class of Straight Chain Fatty Acids. These are fatty acids with a straight aliphatic chain. (inferred from compound structure)Caprylic acid is invovled in Biosynthesis of alkaloids derived from terpenoid and polyketide, Biosynthesis of plant secondary metabolites, and Fatty acid biosynthesis. (KEGG)Caprylic acid is the common name for the eight-carbon saturated fatty acid known by the systematic name octanoic acid. It is found naturally in the milk of various mammals, and it is a minor constituent of coconut oil and palm kernel oil. It is an oily liquid that is minimally soluble in water with a slightly unpleasant rancid-like smell and taste. Two other acids are named after goats: caproic (C6) and capric (C10). Along with caprylic acid these total 15% in goat milk fat. (WikiPedia) |
|---|
|
Structure |
|
|---|
| Synonyms: | - (Z)-octadec-9-enoyl phosphate
- (Z)-Octadec-9-enoyl phosphoric acid
- Octadecanoyl-phosphoric acid (N-C18:1)
|
|---|
|
Chemical Formula: |
C18H33O5P |
|---|
| Average Molecular Weight: |
360.4254 |
|---|
| Monoisotopic Molecular
Weight: |
360.206560678 |
|---|
| InChI Key: |
VHHMNNRGNQWMKU-KTKRTIGZSA-L |
|---|
| InChI: | InChI=1S/C18H35O5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)23-24(20,21)22/h9-10H,2-8,11-17H2,1H3,(H2,20,21,22)/p-2/b10-9- |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | [(9Z)-octadec-9-enoyloxy]phosphonate |
|---|
|
Traditional IUPAC Name: |
(9Z)-octadec-9-enoyloxyphosphonate |
|---|
| SMILES: | CCCCCCCC\C=C/CCCCCCCC(=O)OP([O-])([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as organic phosphoric acids. These are organic compounds containing phosphoric acid, with the general structure OP(O)(=O)O. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organophosphorus compounds |
|---|
|
Class |
Organic phosphoric acids and derivatives |
|---|
| Sub Class | Organic phosphoric acids |
|---|
|
Direct Parent |
Organic phosphoric acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Organic phosphate
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Organic anion
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Membrane |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | 20981657 | | Kegg ID | Not Available | | ChemSpider ID | 20482618 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|