|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001646 |
|---|
|
Identification |
|---|
| Name: |
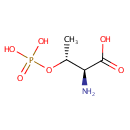
L-Threonine O-3-phosphate |
|---|
| Description: | In Pseudomonas aeruginosa, acid phosphatase / phosphotransferase and alkaline phosphatase are the enzymes that catalyze the chemical reaction L-threonine 3-O-phosphate[periplasmic space] + H2O[periplasmic space] -> L-threonine[periplasmic space] + phosphate[periplasmic space], where L-Threonine O-3-phosphate is a substrate (EcoCyc compound: L-THREONINE-O-3-PHOSPHATE). |
|---|
|
Structure |
|
|---|
| Synonyms: | - (2S,3R)-2-amino-3-hydroxybutanoate
- (2S,3R)-2-amino-3-hydroxybutanoate 3-phosphate
- (2S,3R)-2-amino-3-hydroxybutanoic acid
- (2S,3R)-2-amino-3-hydroxybutanoic acid 3-phosphate
- (2S,3R)-2-amino-3-Hydroxybutanoic acid 3-phosphoric acid
- L-Threonine O-3-phosphate
- L-Threonine O-3-phosphoric acid
- L-Threonine O-phosphate
- L-Threonine O-phosphoric acid
- L-Threonine phosphate
- L-Threonine phosphoric acid
- O-Phospho-L-threonine
- O-Phosphono-L-threonine
- O3-phosphothreonine
- Phospho-L-threonine
- Phosphothreonine
- Synonyms Sources
- Threonine phosphate ester
- Threonine phosphoric acid ester
- Threoninium dihydrogen phosphate
- Threoninium dihydrogen phosphoric acid
|
|---|
|
Chemical Formula: |
C4H10NO6P |
|---|
| Average Molecular Weight: |
199.0991 |
|---|
| Monoisotopic Molecular
Weight: |
199.024573569 |
|---|
| InChI Key: |
USRGIUJOYOXOQJ-GBXIJSLDSA-N |
|---|
| InChI: | InChI=1S/C4H10NO6P/c1-2(3(5)4(6)7)11-12(8,9)10/h2-3H,5H2,1H3,(H,6,7)(H2,8,9,10)/t2-,3+/m1/s1 |
|---|
| CAS
number: |
1114-81-4 |
|---|
| IUPAC Name: | (2S,3R)-2-amino-3-(phosphonooxy)butanoic acid |
|---|
|
Traditional IUPAC Name: |
phosphothreonine |
|---|
| SMILES: | C[C@@H](OP(O)(O)=O)[C@H](N)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
L-alpha-amino acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- L-alpha-amino acid
- Phosphoethanolamine
- Monoalkyl phosphate
- Amino fatty acid
- Fatty acyl
- Fatty acid
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kataoka, H., Nakai, K., Katagiri, Y., Makita, M. (1993). "Analysis of free and bound O-phosphoamino acids in urine by gas chromatography with flame photometric detection." Biomed Chromatogr 7:184-188. Pubmed: 7693088
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|