|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000906 |
|---|
|
Identification |
|---|
| Name: |
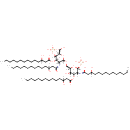
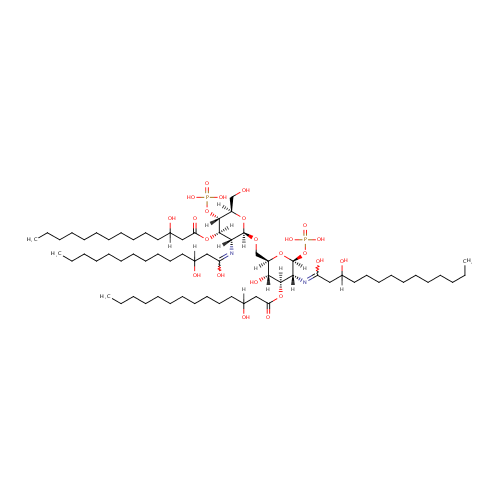
2,3,2'3'-Tetrakis(3-hydroxytetradecanoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate |
|---|
| Description: | 2,3,2'3'-tetrakis(3-hydroxytetradecanoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate is a member of the chemical class known as Dihexoses. These are disaccharides containing two hexose carbohydrates. Pseudomonas aeruginosa KdtA (EcKdtA) is a bifunctional enzyme that transfers two KDO units from two CMP-KDO molecules to lipid IV(A). (PMID 20394418) |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2,3,2'3'-Tetrakis(3-hydroxytetradecanoyl)-D-glucosaminyl-1,6-b-D-glucosamine 1,4'-bisphosphate
- 2,3,2'3'-Tetrakis(3-hydroxytetradecanoyl)-D-glucosaminyl-1,6-b-D-glucosamine 1,4'-bisphosphoric acid
- 2,3,2'3'-Tetrakis(3-hydroxytetradecanoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphoric acid
- 2,3,2'3'-Tetrakis(3-hydroxytetradecanoyl)-D-glucosaminyl-1,6-β-D-glucosamine 1,4'-bisphosphate
- 2,3,2'3'-Tetrakis(3-hydroxytetradecanoyl)-D-glucosaminyl-1,6-β-D-glucosamine 1,4'-bisphosphoric acid
- Lipid a disaccharide bisphosphate
- Lipid a disaccharide bisphosphoric acid
- Lipid IV(a)
|
|---|
|
Chemical Formula: |
C68H130N2O23P2 |
|---|
| Average Molecular Weight: |
1405.7069 |
|---|
| Monoisotopic Molecular
Weight: |
1404.8539615 |
|---|
| InChI Key: |
KVJWZTLXIROHIL-XFXUPMIZSA-N |
|---|
| InChI: | InChI=1S/C68H130N2O23P2/c1-5-9-13-17-21-25-29-33-37-41-51(72)45-57(76)69-61-65(90-59(78)47-53(74)43-39-35-31-27-23-19-15-11-7-3)63(80)56(89-68(61)93-95(84,85)86)50-87-67-62(70-58(77)46-52(73)42-38-34-30-26-22-18-14-10-6-2)66(64(55(49-71)88-67)92-94(81,82)83)91-60(79)48-54(75)44-40-36-32-28-24-20-16-12-8-4/h51-56,61-68,71-75,80H,5-50H2,1-4H3,(H,69,76)(H,70,77)(H2,81,82,83)(H2,84,85,86)/t51?,52?,53?,54?,55-,56-,61-,62-,63-,64-,65-,66-,67-,68+/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | N-[(2S,3R,4R,5S,6R)-6-({[(2R,3R,4R,5S,6R)-3-[(1,3-dihydroxytetradecylidene)amino]-6-(hydroxymethyl)-4-[(3-hydroxytetradecanoyl)oxy]-5-(phosphonooxy)oxan-2-yl]oxy}methyl)-5-hydroxy-4-[(3-hydroxytetradecanoyl)oxy]-2-(phosphonooxy)oxan-3-yl]-3-hydroxytetradecanimidic acid |
|---|
|
Traditional IUPAC Name: |
N-[(2S,3R,4R,5S,6R)-6-({[(2R,3R,4R,5S,6R)-3-[(1,3-dihydroxytetradecylidene)amino]-6-(hydroxymethyl)-4-[(3-hydroxytetradecanoyl)oxy]-5-(phosphonooxy)oxan-2-yl]oxy}methyl)-5-hydroxy-4-[(3-hydroxytetradecanoyl)oxy]-2-(phosphonooxy)oxan-3-yl]-3-hydroxytetradecanimidic acid |
|---|
| SMILES: | [H]C(O)(CCCCCCCCCCC)CC(=O)O[C@@]1([H])[C@]([H])(O)[C@@]([H])(CO[C@]2([H])O[C@]([H])(CO)[C@@]([H])(OP(O)(O)=O)[C@]([H])(OC(=O)CC([H])(O)CCCCCCCCCCC)[C@@]2([H])N=C(O)CC([H])(O)CCCCCCCCCCC)O[C@@]([H])(OP(O)(O)=O)[C@]1([H])N=C(O)CC([H])(O)CCCCCCCCCCC |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as saccharolipids. These are compounds in which fatty acids are linked directly to a sugar backbone, forming structures that are compatible with membrane bilayers. In the saccharolipids, a sugar substitutes for the glycerol backbone that is present in glycerolipids and glycerophospholipids. The most familiar saccharolipids contain an acylated glucosamine. In contrast to others glycolipids, the fatty acid is not glycosidically linked to the sugar moiety. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Saccharolipids |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Saccharolipids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Saccharolipid
- N-acyl-alpha-hexosamine
- Disaccharide phosphate
- Glucosamine
- Amino sugar
- O-glycosyl compound
- Glycosyl compound
- Disaccharide
- Monoalkyl phosphate
- Amino saccharide
- Fatty acid ester
- Beta-hydroxy acid
- Fatty acyl
- Alkyl phosphate
- Phosphoric acid ester
- Oxane
- Organic phosphoric acid derivative
- Organic phosphate
- Hydroxy acid
- Dicarboxylic acid or derivatives
- Secondary alcohol
- Carboxylic acid ester
- Oxacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboxylic acid derivative
- Carboximidic acid derivative
- Carboximidic acid
- Acetal
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
- Tetraacylaminosugars (C04919 )
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Membrane |
|---|
| Reactions: | Adenosine triphosphate + 2,3,2',3'-Tetrakis(3-hydroxytetradecanoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1-phosphate <> ADP + 2,3,2'3'-Tetrakis(3-hydroxytetradecanoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate2,3,2'3'-Tetrakis(3-hydroxytetradecanoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate + CMP-3-Deoxy-D-manno-octulosonate + 2,3,2'3'-Tetrakis(beta-hydroxymyristoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate <> KDO-lipid IV(A) + Cytidine monophosphateUndecaprenyl phosphate alpha-L-Ara4N + 2,3,2'3'-Tetrakis(3-hydroxytetradecanoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate <> Lipid IIA + Di-trans,poly-cis-undecaprenyl phosphate |
|---|
|
Pathways: |
- Lipopolysaccharide biosynthesis pae00540
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Chung, H. S., Raetz, C. R. (2010). "Interchangeable domains in the Kdo transferases of Escherichia coli and Haemophilus influenzae." Biochemistry 49:4126-4137. Pubmed: 20394418
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|