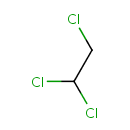
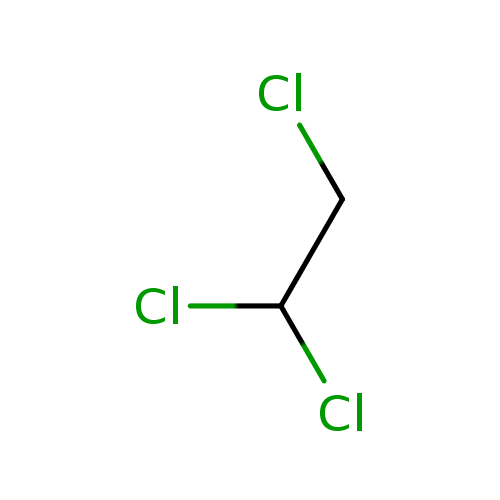
2,2-Dichloroacetaldehyde (PAMDB000902)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000902 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 2,2-Dichloroacetaldehyde | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 2,2-dichloroacetaldehyde is a member of the chemical class known as Organochlorides. These are organic compounds containing a chlorine atom. N2,3-Ethenoguanine (epsilon G) is a product of vinyl chloride reaction with DNA in vivo and of its ultimate metabolite, chloroacetaldehyde, in vitro. (PMID 2013138). It is a bifunctional compound, making it a useful building block chemical. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C2H3Cl3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 133.404 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 131.930033217 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | UBOXGVDOUJQMTN-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C2H3Cl3/c3-1-2(4)5/h2H,1H2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 79-02-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 1,1,2-trichloroethane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | 1,1, 2-trichloroethane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | ClCC(Cl)Cl | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as organochlorides. These are compounds containing a chemical bond between a carbon atom and a chlorine atom. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organohalogen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organochlorides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Organochlorides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Liquid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | -37.5 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Not Available
- Specific function:
- Not Available
- Gene Name:
- yfcG
- Locus Tag:
- PA1033
- Molecular weight:
- 24.5 kDa