|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000850 |
|---|
|
Identification |
|---|
| Name: |
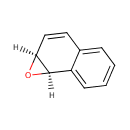
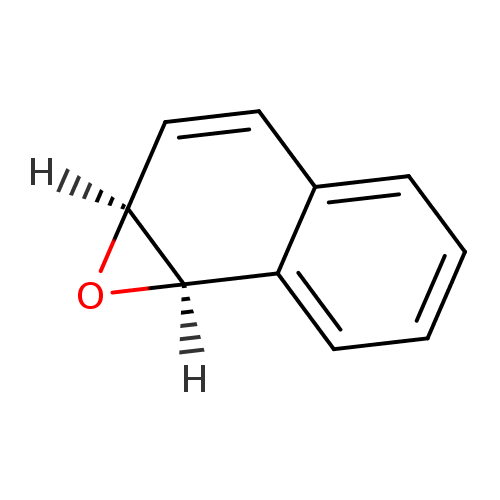
(1R,2S)-Naphthalene 1,2-oxide |
|---|
| Description: | (1r,2s)-naphthalene 1,2-oxide belongs to the class of Naphthalenes. These are compounds containing a naphthalene moiety, which consists of two fused benzene rings. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (1R,2S)-Naphthalene epoxide
- (1R,2S,4R)-(+)-borneol
- 1a,7b-dihydro-(1AS,7BR)-naphth(1,2-b)oxirene
- 1alpha,7beta-dihydro-(1aS,7bR)-Naphth(1,2-b)oxirene
- 1α,7β-dihydro-(1AS,7BR)-naphth(1,2-b)oxirene
- Borneocamphor
- D-borneol
- Endo-2-bornanol
- Sumatra camphor
|
|---|
|
Chemical Formula: |
C10H8O |
|---|
| Average Molecular Weight: |
144.1699 |
|---|
| Monoisotopic Molecular
Weight: |
144.057514878 |
|---|
| InChI Key: |
XQIJIALOJPIKGX-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C10H8O/c1-2-4-8-7(3-1)5-6-9-10(8)11-9/h1-6,9-10H |
|---|
| CAS
number: |
73136-20-6 |
|---|
| IUPAC Name: | (1aR,7aS)-1aH,7aH-naphtho[1,2-b]oxirene |
|---|
|
Traditional IUPAC Name: |
(1aR,7aS)-1aH,7aH-naphtho[1,2-b]oxirene |
|---|
| SMILES: | O1C2C=CC3=CC=CC=C3C12 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as naphthalenes. These are compounds containing a naphthalene moiety, which consists of two fused benzene rings. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
|
Class |
Naphthalenes |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Naphthalenes |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Naphthalene
- Oxacycle
- Organoheterocyclic compound
- Ether
- Oxirane
- Dialkyl ether
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|