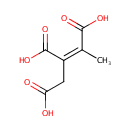
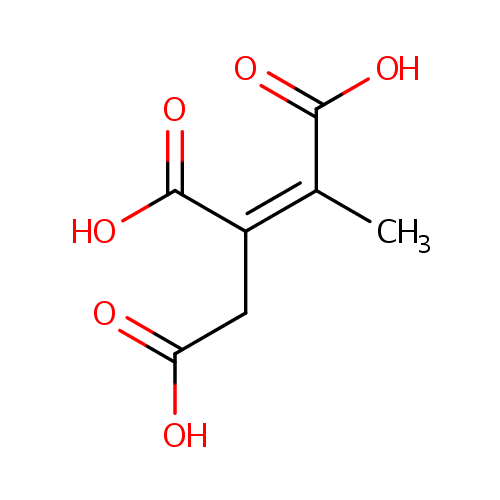
Cis-2-Methylaconitate (PAMDB000668)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000668 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Cis-2-Methylaconitate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | cis-2-Methylaconitate is produced due to the dehydration of 2-methylcitrate in 2-methylcitric acid cycle. The cycle is catalyzed by a cofactor-less (PrpD) enzyme or by an aconitase-like (AcnD) enzyme. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C7H8O6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 188.1348 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 188.032087988 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | NUZLRKBHOBPTQV-ARJAWSKDSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C7H8O6/c1-3(6(10)11)4(7(12)13)2-5(8)9/h2H2,1H3,(H,8,9)(H,10,11)(H,12,13)/b4-3- | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 6061-93-4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (1Z)-1-methylprop-1-ene-1,2,3-tricarboxylic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | α-methylaconitate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | C\C(C(O)=O)=C(/CC(O)=O)C(O)=O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as tricarboxylic acids and derivatives. These are carboxylic acids containing exactly three carboxyl groups. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carboxylic acids and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Tricarboxylic acids and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Tricarboxylic acids and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Cis-2-Methylaconitate + Water <> Methylisocitric acid Methylcitric acid + (2S,3S)-2-hydroxybutane-1,2,3-tricarboxylate <> Cis-2-Methylaconitate + Water Methylcitric acid <> Cis-2-Methylaconitate + Water (2S,3S)-2-hydroxybutane-1,2,3-tricarboxylate > Cis-2-Methylaconitate + Water 2-Methylcitric acid + Methylcitric acid > Water + Cis-2-Methylaconitate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Tabuchi, Takeshi; Uchiyama, Hiroo. Methylcitrate condensing and methylisocitrate cleaving enzymes. Evidence for the pathway of oxidation of propionyl-CoA to pyruvate via C7-tricarboxylic acids. Agricultural and Biological Chemistry (1975), 39(10), | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||