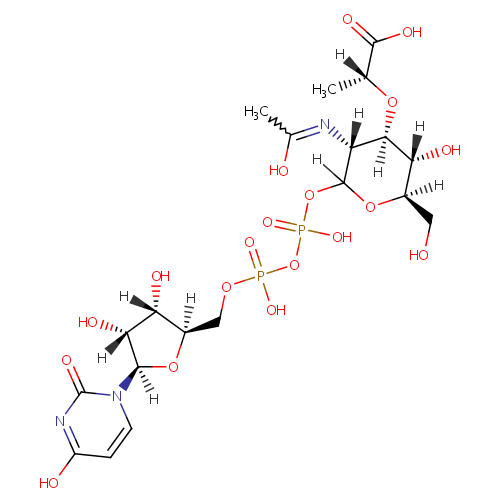
UDP-N-Acetylmuraminate (PAMDB000485)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000485 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | UDP-N-Acetylmuraminate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | UDP-N-acetylmuaminate is nucleoside diphosphate sugar which is formed from UDP-N-acetylglucosamine and phosphoenolpyruvate. It is a key component of peptidoglycan synthesis. The peptidoglycan synthesis pathway starts at the cytoplasm, where in six steps the peptidoglycan precursor a UDP-N-acetylmuramoyl-pentapeptide is synthesized. This precursor is then attached to the memberane acceptor all-trans-undecaprenyl phosphate, generating a N-acetylmuramoyl-pentapeptide-diphosphoundecaprenol, also known as lipid I. Another transferase then adds UDP-N-acetyl-alpha-D-glucosamine, yielding the complete monomeric unit a lipid , also known as lipid . This final lipid intermediate is transferred through the membrane. The peptidoglycan monomers are then polymerized on the outside surface by glycosyltransferases, which form the linear glycan chains, and transpeptidases, which catalyze the formation of peptide crosslinks. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C20H31N3O19P2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 679.4164 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 679.102698849 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | NQBRVZNDBBMBLJ-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C20H31N3O19P2/c1-7(18(30)31)38-16-12(21-8(2)25)19(40-9(5-24)14(16)28)41-44(35,36)42-43(33,34)37-6-10-13(27)15(29)17(39-10)23-4-3-11(26)22-20(23)32/h3-4,7,9-10,12-17,19,24,27-29H,5-6H2,1-2H3,(H,21,25)(H,30,31)(H,33,34)(H,35,36)(H,22,26,32) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2R)-2-{[(3R,4R,5S,6R)-2-({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(4-hydroxy-2-oxo-1,2-dihydropyrimidin-1-yl)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-5-hydroxy-3-[(1-hydroxyethylidene)amino]-6-(hydroxymethyl)oxan-4-yl]oxy}propanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | (2R)-2-{[(3R,4R,5S,6R)-2-[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(4-hydroxy-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]-5-hydroxy-3-[(1-hydroxyethylidene)amino]-6-(hydroxymethyl)oxan-4-yl]oxy}propanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC(OC1C(O)C(CO)OC(OP(O)(=O)OP(O)(=O)OCC2OC(C(O)C2O)N2C=CC(=O)NC2=O)C1NC(C)=O)C(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as pyrimidine nucleotide sugars. These are pyrimidine nucleotides bound to a saccharide derivative through the terminal phosphate group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Nucleosides, nucleotides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Pyrimidine nucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pyrimidine nucleotide sugars | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyrimidine nucleotide sugars | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | L-Alanine + Adenosine triphosphate + UDP-N-Acetylmuraminate <> ADP + Hydrogen ion + Phosphate + UDP-N-Acetylmuramoyl-L-alanine Hydrogen ion + NADPH + UDP-N-Acetyl-3-(1-carboxyvinyl)-D-glucosamine <> NADP + UDP-N-Acetylmuraminate L-alanine-D-glutamate-meso-2,6-diaminoheptanedioate + Adenosine triphosphate + UDP-N-Acetylmuraminate > ADP + Hydrogen ion + Phosphate + UDP-N-Acetylmuramoyl-L-alanyl-D-glutamyl-meso-2,6-diaminoheptanedioate UDP-N-Acetylmuraminate + NAD <> UDP-N-Acetyl-3-(1-carboxyvinyl)-D-glucosamine + NADH + Hydrogen ion Adenosine triphosphate + UDP-N-Acetylmuraminate + L-Alanine <> ADP + Phosphate + UDP-N-Acetylmuramoyl-L-alanine UDP-N-Acetylmuraminate + L-Ala-D-Glu-meso-A2pm + Adenosine triphosphate > Hydrogen ion + UDP-N-Acetylmuramoyl-L-alanyl-D-glutamyl-meso-2,6-diaminoheptanedioate + ADP + Phosphate UDP-N-Acetylmuraminate + NADP > UDP-N-Acetyl-3-(1-carboxyvinyl)-D-glucosamine + NADPH Adenosine triphosphate + UDP-N-Acetylmuraminate + L-Alanine > ADP + Inorganic phosphate + UDP-N-Acetylmuramoyl-L-alanine UDP-N-acetylmuraminate + Adenosine triphosphate + L-Alanine + UDP-N-Acetylmuraminate + L-Alanine > UDP-N-Acetylmuramoyl-L-alanine + Adenosine diphosphate + Phosphate + ADP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in flavin adenine dinucleotide binding
- Specific function:
- Cell wall formation
- Gene Name:
- murB
- Locus Tag:
- PA2977
- Molecular weight:
- 37.6 kDa
Reactions
| UDP-N-acetylmuramate + NADP(+) = UDP-N-acetyl-3-O-(1-carboxyvinyl)-D-glucosamine + NADPH. |
- General function:
- Involved in ATP binding
- Specific function:
- Cell wall formation
- Gene Name:
- murC
- Locus Tag:
- PA4411
- Molecular weight:
- 51.9 kDa
Reactions
| ATP + UDP-N-acetylmuramate + L-alanine = ADP + phosphate + UDP-N-acetylmuramoyl-L-alanine. |
- General function:
- Involved in ATP binding
- Specific function:
- Reutilizes the intact tripeptide L-alanyl-gamma-D- glutamyl-meso-diaminopimelate by linking it to UDP-N-acetylmuramic acid
- Gene Name:
- mpl
- Locus Tag:
- PA4020
- Molecular weight:
- 48.5 kDa