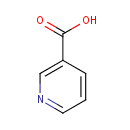
Nicotinic acid (PAMDB000399)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000399 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Nicotinic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Nicotinic acid, also known as niacin or vitamin B3, is a derivative of pyridine, with a carboxyl group (COOH) at the 3-position. Other forms of vitamin B3 include the corresponding amide, nicotinamide ("niacinamide"), where the carboxyl group has been replaced by a carboxamide group (CONH2), as well as more complex amides and a variety of esters. The uptake of nicotinic acid by Pseudomonas aeruginosa is dependent on the presence of the enzyme nicotinic acid phosphoribosyl transferase and a source of energy. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C6H5NO2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 123.1094 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 123.032028409 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | PVNIIMVLHYAWGP-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 59-67-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | pyridine-3-carboxylic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | niacin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | OC(=O)C1=CN=CC=C1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as pyridinecarboxylic acids. These are compounds containing a pyridine ring bearing a carboxylic acid group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Pyridines and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pyridinecarboxylic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyridinecarboxylic acids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 236.6 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosine triphosphate + Water + Nicotinic acid + Phosphoribosyl pyrophosphate > ADP + Nicotinamide ribotide + Phosphate + Pyrophosphate Water + Niacinamide > Nicotinic acid + Ammonium Dimethylbenzimidazole + Nicotinamide ribotide <> N1-(5-Phospho-a-D-ribosyl)-5,6-dimethylbenzimidazole + Hydrogen ion + Nicotinic acid Niacinamide + Water <> Nicotinic acid + Ammonia Niacinamide + Water > Hydrogen ion + Nicotinic acid + Ammonia Nicotinamide ribotide + Pyrophosphate < Hydrogen ion + Nicotinic acid + Phosphoribosyl pyrophosphate Nicotinamide ribotide + Dimethylbenzimidazole > Nicotinic acid + N1-(5-Phospho-a-D-ribosyl)-5,6-dimethylbenzimidazole Nicotinamide ribotide + Pyrophosphate > Nicotinic acid + Phosphoribosyl pyrophosphate Nicotinic acid + Water + Adenosine triphosphate + Phosphoribosyl pyrophosphate > Phosphate + Adenosine diphosphate + Pyrophosphate + nicotinate beta-D-ribonucleotide + ADP + Nicotinamide ribotide Dimethylbenzimidazole + nicotinate beta-D-ribonucleotide + Nicotinamide ribotide > Hydrogen ion + Nicotinic acid + N1-(5-Phospho-a-D-ribosyl)-5,6-dimethylbenzimidazole | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | McElvain, S. M.; Goese, M. A. Preparation of nicotinic acid from pyridine. Journal of the American Chemical Society (1941), 63 2283-4. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in nicotinate phosphoribosyltransferase activity
- Specific function:
- Nicotinate D-ribonucleotide + diphosphate = nicotinate + 5-phospho-alpha-D-ribose 1-diphosphate
- Gene Name:
- pncB
- Locus Tag:
- PA4919
- Molecular weight:
- 46.1 kDa
Reactions
| Beta-nicotinate D-ribonucleotide + diphosphate = nicotinate + 5-phospho-alpha-D-ribose 1-diphosphate. |