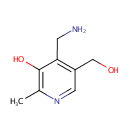
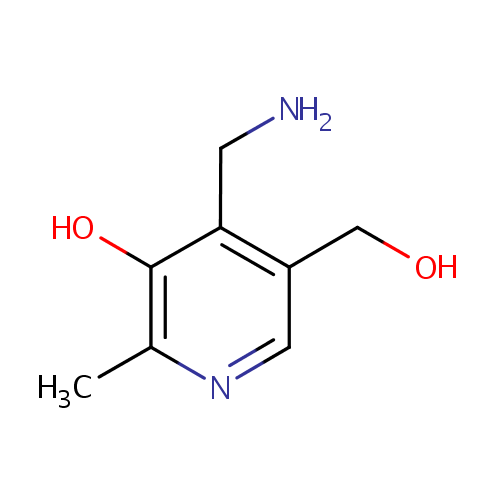
| References: |
- Berzas Nevado JJ, Murillo Pulgarin JA, Gomez Laguna MA: Determination of pyridoxamine in urine by matrix isopotential synchronous fluorescence spectrometry. Analyst. 1995 Jan;120(1):171-4. Pubmed: 7710125
- Esteve-Romero J, Capella-Peiro ME, Monferrer-Pons L, Gil-Agusti M: Micellar liquid chromatography in clinical chemistry: application to the monitorization of B6 vitamins. Clin Chim Acta. 2004 Oct;348(1-2):69-77. Pubmed: 15369738
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Rokitzki L, Sagredos AN, Reuss F, Buchner M, Keul J: Acute changes in vitamin B6 status in endurance athletes before and after a marathon. Int J Sport Nutr. 1994 Jun;4(2):154-65. Pubmed: 8054960
- Sharma SK, Dakshinamurti K: Determination of vitamin B6 vitamers and pyridoxic acid in biological samples. J Chromatogr. 1992 Jul 1;578(1):45-51. Pubmed: 1400785
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. Pubmed: 19212411
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|