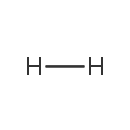
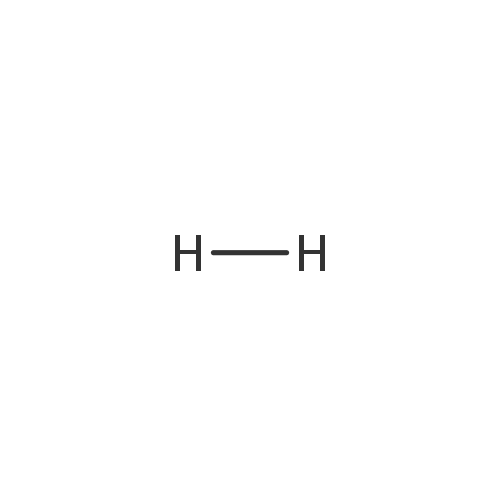
Hydrogen (gas) (PAMDB000355)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000355 | ||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||
| Name: | Hydrogen (gas) | ||||||||||||||||||||||||||||||||||||||||||
| Description: | Hydrogen is a colorless, odorless, nonmetallic, tasteless, highly flammable diatomic gas with the molecular formula H2. With an atomic weight of 1.00794, hydrogen is the lightest element. Besides the common H1 isotope, hydrogen exists as the stable isotope Deuterium and the unstable, radioactive isotope Tritium. Hydrogen is the most abundant of the chemical elements, constituting roughly 75% of the universe's elemental mass. Hydrogen can form compounds with most elements and is present in water and most organic compounds. It plays a particularly important role in acid-base chemistry, in which many reactions involve the exchange of protons between soluble molecules. Oxidation of hydrogen, in the sense of removing its electron, formally gives H+, containing no electrons and a nucleus which is usually composed of one proton. That is why H+ is often called a proton. This species is central to discussion of acids. Under the Bronsted-Lowry theory, acids are proton donors, while bases are proton acceptors. The transport of protons or hydrogen ions across the membrane(ie the creation of a proton gradient) is critical to the generation of energy (ATP) for all living cells. | ||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | H2 | ||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 2.0159 | ||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 2.015650064 | ||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | UFHFLCQGNIYNRP-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/H2/h1H | ||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 1333-74-0 | ||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [H][H] | ||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of inorganic compounds known as other non-metal hydrides. These are inorganic compounds in which the heaviest atom bonded to a hydrogen atom is belongs to the class of 'other non-metals'. | ||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Inorganic compounds | ||||||||||||||||||||||||||||||||||||||||||
| Super Class | Homogeneous non-metal compounds | ||||||||||||||||||||||||||||||||||||||||||
| Class | Other non-metal organides | ||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Other non-metal hydrides | ||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Other non-metal hydrides | ||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||
| State: | Liquid | ||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||
| Melting point: | -259.2 °C | ||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 2 Hydrogen ion + Hydrogen (gas) + Ubiquinone-8 > Ubiquinol-8 +2 Hydrogen ion 2-Demethylmenaquinone 8 + 2 Hydrogen ion + Hydrogen (gas) > 2-Demethylmenaquinol 8 +2 Hydrogen ion 2 Hydrogen ion + Hydrogen (gas) + Menaquinone 8 > Menaquinol 8 +2 Hydrogen ion Formic acid + Hydrogen ion > Carbon dioxide + Hydrogen (gas) Water + Phosphonate > Hydrogen (gas) + Phosphate Hydrogen ion > Hydrogen (gas) a menaquinone + Hydrogen ion + Hydrogen (gas) > a menaquinol + Hydrogen ion Hydrogen (gas) + A > AH(2) Hydrogen (gas) + Acceptor <> Reduced acceptor | ||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Ait-Ichou, I.; Formenti, M.; Pommier, B.; Teichner, S. J. Photocatalytic dehydrogenation of isopropanol on platinum/titania catalysts. Journal of Catalysis (1985), 91(2), 293-307. | ||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in catalytic activity
- Specific function:
- A phosphate monoester + H(2)O = an alcohol + phosphate
- Gene Name:
- phoA
- Locus Tag:
- PA3296
- Molecular weight:
- 50.4 kDa
Reactions
| A phosphate monoester + H(2)O = an alcohol + phosphate. |