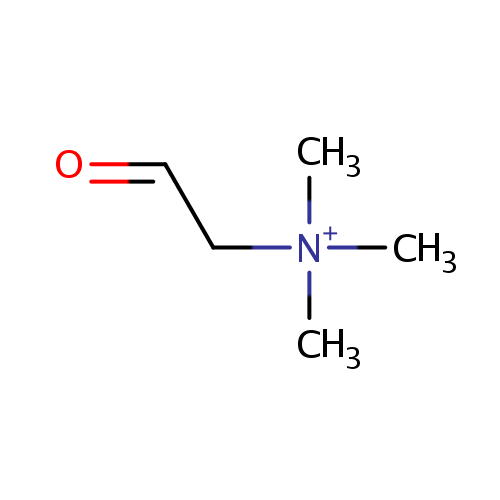
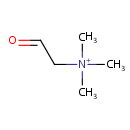Betaine aldehyde (PAMDB000309)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000309 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Betaine aldehyde | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Betaine aldehyde is an intermediate in the metabolism of glycine, serine and threonine. Betaine aldehyde dehydrogenase facilitates the conversion of betaine aldehyde to betaine. (PMID: 12467448, 7646513) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C5H12NO | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 102.1549 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 102.091889011 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | SXKNCCSPZDCRFD-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C5H12NO/c1-6(2,3)4-5-7/h5H,4H2,1-3H3/q+1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 7418-61-3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | trimethyl(2-oxoethyl)azanium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | betaine aldehyde | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | C[N+](C)(C)CC=O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as quaternary ammonium salts. These are compounds containing positively charged polyatomic ion of the structure NR4+, R being an alkyl group or an aryl group. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organonitrogen compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Quaternary ammonium salts | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Quaternary ammonium salts | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Choline + NAD > Betaine aldehyde + Hydrogen ion + NADH Betaine aldehyde + Water + NADP > Betaine +2 Hydrogen ion + NADPH Betaine aldehyde + Water + NAD <> Betaine +2 Hydrogen ion + NADH Choline + Acceptor + Acceptor <> Betaine aldehyde + Reduced acceptor + Reduced acceptor Choline + an oxidized electron acceptor > Betaine aldehyde + a reduced electron acceptor Choline + acceptor > Betaine aldehyde + reduced acceptor Betaine aldehyde + NAD + Water > Betaine + NADH alkylsulfonate + FMNH2 + Oxygen > Betaine aldehyde + Sulfite + Flavin Mononucleotide + Water +2 Hydrogen ion + Sulfite Butanesulfonate + Oxygen + FMNH2 > Hydrogen ion + Water + Sulfite + Flavin Mononucleotide + Betaine aldehyde + Sulfite Oxygen + FMNH2 + 3-(N-morpholino)propanesulfonate > Sulfite + Water + Hydrogen ion + Flavin Mononucleotide + Betaine aldehyde + Sulfite ethanesulfonate + Oxygen + FMNH2 > Hydrogen ion + Water + Flavin Mononucleotide + Sulfite + Betaine aldehyde + Sulfite isethionate + Oxygen + FMNH2 > Betaine aldehyde + Flavin Mononucleotide + Hydrogen ion + Water + Sulfite + Sulfite Oxygen + methanesulfonate + FMNH2 + Methanesulfonate > Hydrogen ion + Water + Flavin Mononucleotide + Sulfite + Betaine aldehyde + Sulfite | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Cromwell, B. T.; Rennie, S. D. Biosynthesis and metabolism of betaines in plants. II. Biosynthesis of glycinebetaine(betaine) in higher plants. Biochemical Journal (1954), 58 318-22. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||