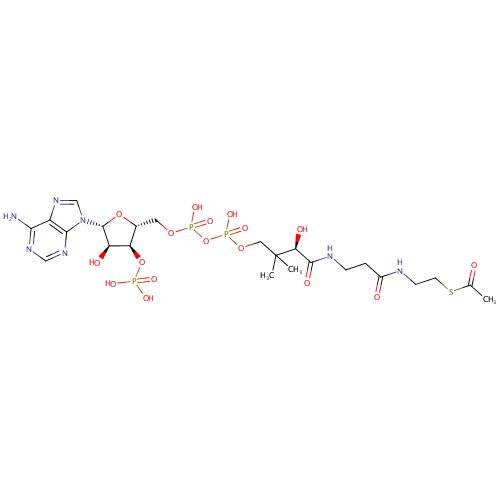
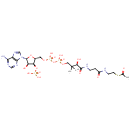Acetyl-CoA (PAMDB000298)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000298 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Acetyl-CoA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Acetyl-CoA is the thioester formed between coenzyme A (a thiol) and acetic acid (an acyl group carrier). Acetyl-CoA is produced during the second step of aerobic cellular respiration, pyruvate decarboxylation. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for energy production. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C23H38N7O17P3S | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 809.571 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 809.125773051 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | ZSLZBFCDCINBPY-ZSJPKINUSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C23H38N7O17P3S/c1-12(31)51-7-6-25-14(32)4-5-26-21(35)18(34)23(2,3)9-44-50(41,42)47-49(39,40)43-8-13-17(46-48(36,37)38)16(33)22(45-13)30-11-29-15-19(24)27-10-28-20(15)30/h10-11,13,16-18,22,33-34H,4-9H2,1-3H3,(H,25,32)(H,26,35)(H,39,40)(H,41,42)(H2,24,27,28)(H2,36,37,38)/t13-,16-,17-,18+,22-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 72-89-9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[(2R,3S,4R,5R)-2-({[({[(3R)-3-[(2-{[2-(acetylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]-3-hydroxy-2,2-dimethylpropoxy](hydroxy)phosphoryl}oxy)(hydroxy)phosphoryl]oxy}methyl)-5-(6-amino-9H-purin-9-yl)-4-hydroxyoxolan-3-yl]oxy}phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | acetyl-CoA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC(=O)SCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as acyl coas. These are organic compounds containing a coenzyme A substructure linked to an acyl chain. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Fatty Acyls | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Fatty acyl thioesters | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Acyl CoAs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Coenzyme A + 2 flavodoxin semi oxidized + Pyruvic acid <> Acetyl-CoA + Carbon dioxide +2 Flavodoxin reduced + Hydrogen ion Coenzyme A + Pyruvic acid <> Acetyl-CoA + Formic acid Coenzyme A + NAD + Pyruvic acid > Acetyl-CoA + Carbon dioxide + NADH Acetyl-CoA + Adenosine triphosphate + Hydrogen carbonate <> ADP + Hydrogen ion + Malonyl-CoA + Phosphate Acetaldehyde + Coenzyme A + NAD <> Acetyl-CoA + Hydrogen ion + NADH acyl carrier protein + Acetyl-CoA <> Acetyl-ACP + Coenzyme A 3-Oxo-5,6-dehydrosuberyl-CoA + Coenzyme A <> 5-Carboxy-2-pentenoyl-CoA + Acetyl-CoA Acetoacetic acid + Acetyl-CoA > Acetoacetyl-CoA + Acetic acid Acetyl-CoA + Butyric acid > Acetic acid + Butyryl-CoA Acetyl-CoA + Hexanoate (N-C6:0) > Acetic acid + Hexanoyl-CoA 2 Acetyl-CoA <> Acetoacetyl-CoA + Coenzyme A Acetyl-CoA + Phosphate <> Acetylphosphate + Coenzyme A Acetyl-CoA + Octanoyl-CoA <> 3-Oxodecanoyl-CoA + Coenzyme A Acetyl-CoA + Butyryl-CoA <> 3-Oxohexanoyl-CoA + Coenzyme A Acetyl-CoA + Tetradecanoyl-CoA <> 3-Oxohexadecanoyl-CoA + Coenzyme A Acetyl-CoA + Hexanoyl-CoA <> 3-Oxooctanoyl-CoA + Coenzyme A Acetyl-CoA + Lauroyl-CoA <> 3-Oxotetradecanoyl-CoA + Coenzyme A Acetyl-CoA + Decanoyl-CoA (N-C10:0CoA) <> 3-Oxododecanoyl-CoA + Coenzyme A 3-Oxooctadecanoyl-CoA + Coenzyme A <> Acetyl-CoA + Palmityl-CoA Acetyl-CoA + Glyoxylic acid + Water <> Coenzyme A + Hydrogen ion + L-Malic acid alpha-Ketoisovaleric acid + Acetyl-CoA + Water + a-Ketoisovaleric acid <> 2-Isopropylmalic acid + Coenzyme A + Hydrogen ion Acetyl-CoA + D-Glucose <> 6-Acetyl-D-glucose + Coenzyme A Acetyl-CoA + D-Maltose <> Acetyl-maltose + Coenzyme A Acetyl-CoA + Water + Oxalacetic acid <> Citric acid + Coenzyme A + Hydrogen ion Acetyl-CoA + Hydrogen ion + Malonyl-[acyl-carrier protein] > Acetoacetyl-ACP + Carbon dioxide + Coenzyme A Coenzyme A + 3-Oxoadipyl-CoA <> Acetyl-CoA + Succinyl-CoA More...Acetyl-CoA + 2-Aminobenzoic acid > N-Acetylanthranilate + Coenzyme A Acetyl-CoA + Spermidine > N1-Acetylspermidine + Coenzyme A + Hydrogen ion Acetyl-CoA + Spermidine > Coenzyme A + Hydrogen ion + N8-Acetylspermidine Acetyl-CoA + Rhamanosyl-N-acetylglucosamyl-undecaprenyl diphosphate > O-Acetyl-rhamanosyl-N-acetylglucosamyl-undecaprenyl diphosphate + Coenzyme A Acetyl-CoA + L-Glutamate <> N-Acetyl-L-alanine + Coenzyme A + Hydrogen ion + N-Acetylglutamic acid Acetyl-CoA + L-Serine <> O-Acetylserine + Coenzyme A Acetyl-CoA + Glycine <> L-2-Amino-3-oxobutanoic acid + Coenzyme A Acetyl-CoA + N-Acetyl-glucosamine 1-phosphate > N-Acetyl-glucosamine 1-phosphate + Coenzyme A + Hydrogen ion Acetyl-CoA + dTDP-D-Fucosamine > Coenzyme A + dTDP-4-Acetamido-4,6-dideoxy-D-galactose + Hydrogen ion Acetic acid + Adenosine triphosphate + Coenzyme A <> Acetyl-CoA + Adenosine monophosphate + Pyrophosphate Acetyl-CoA + L-Glutamate <> Coenzyme A + N-Acetyl-L-alanine Citric acid + Coenzyme A <> Acetyl-CoA + Water + Oxalacetic acid L-Malic acid + Coenzyme A <> Acetyl-CoA + Water + Glyoxylic acid Adenosine triphosphate + Acetyl-CoA + Hydrogen carbonate <> ADP + Phosphate + Malonyl-CoA Propionyl-CoA + Acetyl-CoA <> Coenzyme A + 2-Methylacetoacetyl-CoA Acetyl-CoA + Putrescine <> Coenzyme A + N-Acetylputrescine Acetyl-CoA + Butanoyl-CoA <> Coenzyme A + 3-Oxohexanoyl-CoA Butanoyl-CoA + Acetic acid <> Butyric acid + Acetyl-CoA 2 Reduced ferredoxin + Acetyl-CoA + Carbon dioxide + 2 Hydrogen ion + Oxidized ferredoxin <>2 Oxidized ferredoxin + Pyruvic acid + Coenzyme A + Reduced ferredoxin 2-Isopropylmalic acid + Coenzyme A <> Acetyl-CoA + alpha-Ketoisovaleric acid + Water Acetyl-CoA + Acyl-carrier protein <> Coenzyme A + Acetyl-[acyl-carrier protein] Acetyl-CoA + Enzyme N6-(dihydrolipoyl)lysine + Enzyme N6-(dihydrolipoyl)lysine <> Coenzyme A + [Dihydrolipoyllysine-residue acetyltransferase] S-acetyldihydrolipoyllysine Acetyl-CoA + Carboxybiotin-carboxyl-carrier protein <> Malonyl-CoA + Holo-[carboxylase] Acetyl-CoA + alpha-D-Glucosamine 1-phosphate <> Coenzyme A + Glucosamine-1P 3-Hydroxy-5-oxohexanoate + Acetyl-CoA <> 3-Hydroxy-5-oxohexanoyl-CoA + Acetic acid 7-Methyl-3-oxo-6-octenoyl-CoA + Coenzyme A <> 5-Methylhex-4-enoyl-CoA + Acetyl-CoA 5-Methyl-3-oxo-4-hexenoyl-CoA + Coenzyme A <> 3-Methylcrotonyl-CoA + Acetyl-CoA Demethylphosphinothricin + Acetyl-CoA <> N-Acetyldemethylphosphinothricin + Coenzyme A L-Methionine + Acetyl-CoA N-α-acetyl-L-methionine + Coenzyme A alpha-Ketoisovaleric acid + Acetyl-CoA + Water > Hydrogen ion + 3-Carboxy-3-hydroxy-isocaproate + Coenzyme A an <i>N</i>-hydroxy-arylamine + Acetyl-CoA <> an <i>N</i>-acetoxyarylamine + Coenzyme A Glucosamine-1P + Acetyl-CoA > Hydrogen ion + <i>N</i>-acetyl-α-D-glucosamine 1-phosphate + Coenzyme A a 2,3,4-saturated fatty acyl CoA + Acetic acid <> a fatty acid + Acetyl-CoA Glycine + Acetyl-CoA <> Hydrogen ion + L-2-Amino-3-oxobutanoic acid + Coenzyme A an aliphatic α,ω-diamine + Acetyl-CoA <> an aliphatic <i>N</i>-acetyl-diamine + Coenzyme A + Hydrogen ion Acetyl-CoA + Dihydrolipoamide Coenzyme A + S-Acetyldihydrolipoamide a β-D-galactoside + Acetyl-CoA <> a 6-acetyl-β-D-galactoside + Coenzyme A a 2,3,4-saturated fatty acyl CoA + Acetyl-CoA < a 3-oxoacyl-CoA + Coenzyme A L-Glutamate + Acetyl-CoA <> Hydrogen ion + <i>N</i>-acetyl-L-glutamate + Coenzyme A 3-Oxo-5,6-dehydrosuberyl-CoA + Coenzyme A > 2,3-dehydroadipyl-CoA + Acetyl-CoA Adenosine triphosphate + Acetyl-CoA + Carbonic acid > ADP + Inorganic phosphate + Malonyl-CoA Acyl-CoA + Acetic acid > a fatty acid anion + Acetyl-CoA Acetyl-CoA + Citric acid > Acetic acid + (3S)-Citryl-CoA (3S)-Citryl-CoA > Acetyl-CoA + Oxalacetic acid Acetyl-CoA + Ribosomal-protein L-alanine <> Coenzyme A + Ribosomal-protein N-acetyl-L-alanine Acetyl-CoA + N-Hydroxyarylamine <> Coenzyme A + N-Acetoxyarylamine Acetyl-CoA + Alkane-alpha,omega-diamine <> Coenzyme A + N-Acetyldiamine Acyl-CoA + Acetic acid <> Fatty acid anion + Acetyl-CoA Acetyl-CoA + dTDP-D-Fucosamine <> Coenzyme A + dTDP-4-acetamido-4,6-dideoxy-alpha-D-galactose Acetyl-CoA + beta-D-Galactoside <> Coenzyme A + 6-Acetyl-beta-D-galactoside Acetyl-CoA + Oxalic acid <> Acetic acid + Oxalyl-CoA Acyl-CoA + Acetyl-CoA <> Coenzyme A + 3-Oxoacyl-CoA Acetyl-CoA + Malonyl-[acyl-carrier protein] <> Acetoacetyl-[acp] + Coenzyme A + Carbon dioxide Acetyl-CoA + 3-Hydroxy-5-oxohexanoate > Acetic acid + 3-Hydroxy-5-oxohexanoyl-CoA + 3-Hydroxy-5-oxohexanoyl-CoA a 3-oxoacyl-CoA? + Coenzyme A > Acetyl-CoA + a 2,3,4-saturated fatty acyl CoA? Acetoacetyl-CoA + Coenzyme A + Acetoacetyl-CoA > Acetyl-CoA + Succinyl-CoA + Succinyl-CoA 3-Oxodecanoyl-CoA + Coenzyme A > Acetyl-CoA + Lauroyl-CoA 3-Oxohexanoyl-CoA + Coenzyme A > Acetyl-CoA + Octanoyl-CoA + Octanoyl-CoA 3-Oxododecanoyl-CoA + Coenzyme A > Acetyl-CoA + Tetradecanoyl-CoA 3-Oxotetradecanoyl-CoA + Coenzyme A > Acetyl-CoA + Palmityl-CoA 3-Oxooctanoyl-CoA + Coenzyme A > Acetyl-CoA + Decanoyl-CoA (n-C10:0CoA) + Decanoyl-CoA (N-C10:0CoA) 3-Oxohexadecanoyl-CoA + Coenzyme A > Acetyl-CoA + Stearoyl-CoA + Stearoyl-CoA 3-Oxooctadecanoyl-CoA + Coenzyme A + 3-Oxooctadecanoyl-CoA > Acetyl-CoA + Stearoyl-CoA + Stearoyl-CoA Coenzyme A + 7-Methyl-3-oxo-6-octenoyl-CoA > Acetyl-CoA + 5-Methylhex-4-enoyl-CoA Coenzyme A + 5-Methyl-3-oxo-4-hexenoyl-CoA > Acetyl-CoA + 3-Methylcrotonyl-CoA 2 Acetyl-CoA <> Acetoacetyl-CoA + Coenzyme A + Acetoacetyl-CoA L-Glutamic acid + Acetyl-CoA + L-Glutamate > Coenzyme A + Hydrogen ion + N-Acetylglutamic acid + N-Acetylglutamic acid Decanoyl-CoA (n-C10:0CoA) + Acetyl-CoA + Decanoyl-CoA (N-C10:0CoA) > 3-Oxododecanoyl-CoA + Coenzyme A Octanoyl-CoA + Acetyl-CoA + Octanoyl-CoA <> 3-Oxodecanoyl-CoA + Coenzyme A Acetyl-CoA + Hexanyl-CoA <> 3-Oxooctanoyl-CoA + Coenzyme A Acetyl-CoA + Butyryl-CoA + Butyryl-CoA > 3-Oxohexanoyl-CoA + Coenzyme A Acetyl-CoA + Hydrogen carbonate + Adenosine triphosphate > Adenosine diphosphate + Phosphate + Hydrogen ion + Malonyl-CoA + ADP + Malonyl-CoA a malonyl-[acp] + Hydrogen ion + Acetyl-CoA > Carbon dioxide + Coenzyme A + acetoacetyl-[acp] Acetyl-CoA + a holo-[acyl-carrier protein] > Coenzyme A + an acetyl-[acp] L-Serine + Acetyl-CoA + L-Serine > Coenzyme A + O-Acetylserine 3-Methyl-2-oxovaleric acid + Water + Acetyl-CoA + 3-Methyl-2-oxovaleric acid > Coenzyme A + Hydrogen ion + 2-Isopropylmalic acid Glyoxylic acid + Water + Acetyl-CoA > Coenzyme A + Hydrogen ion + L-Malic acid + L-Malic acid 3-hydroxy-2,4-pentanedione 5-phosphate + Coenzyme A + 3-hydroxy-2,4-pentanedione 5-phosphate > Acetyl-CoA + Dihydroxyacetone phosphate Glucosamine-1P + Acetyl-CoA + Glucosamine-1P > N-Acetyl-glucosamine 1-phosphate + Coenzyme A + Hydrogen ion + N-Acetyl-glucosamine 1-phosphate Acetyl-CoA + dTDP-thomosamine > TDP-Fuc4NAc + Coenzyme A + Hydrogen ion Acetyl-CoA + L-Glutamic acid + L-Glutamate <> N-Acetyl-L-alanine + Coenzyme A + Hydrogen ion + N-Acetylglutamic acid + N-Acetyl-L-alanine + N-Acetylglutamic acid Acetyl-CoA + Dihydrolipoamide + Dihydrolipoamide <> Coenzyme A + S-Acetyldihydrolipoamide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Tucek, S. The synthesis of acetyl coenzyme A and acetylcholine from citrate and acetate in the nerve endings of mammalian brain. Biochimica et Biophysica Acta, General Subjects (1966), 117(1), 278-80. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||