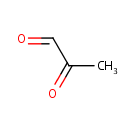
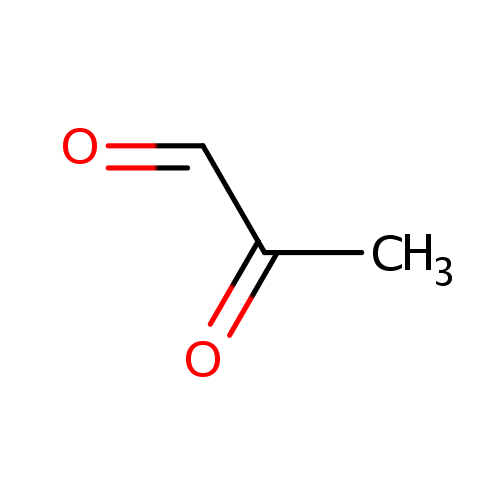
| References: |
- Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW: N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem J. 1997 Jun 1;324 ( Pt 2):565-70. Pubmed: 9182719
- Ahmed N, Dobler D, Dean M, Thornalley PJ: Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J Biol Chem. 2005 Feb 18;280(7):5724-32. Epub 2004 Nov 22. Pubmed: 15557329
- Ahmed N, Thornalley PJ, Dawczynski J, Franke S, Strobel J, Stein G, Haik GM: Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Invest Ophthalmol Vis Sci. 2003 Dec;44(12):5287-92. Pubmed: 14638728
- Baskaran S, Rajan DP, Balasubramanian KA: Formation of methylglyoxal by bacteria isolated from human faeces. J Med Microbiol. 1989 Mar;28(3):211-5. Pubmed: 2926792
- Beisswenger PJ, Drummond KS, Nelson RG, Howell SK, Szwergold BS, Mauer M: Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress. Diabetes. 2005 Nov;54(11):3274-81. Pubmed: 16249455
- Gildersleeve DL, Tobes MC, Natale RB: Rapid analysis for methylglyoxal bis(guanylhydrazone) by reversed-phase ion-pair liquid chromatography. Clin Chem. 1985 Dec;31(12):1979-84. Pubmed: 4064286
- Haik GM Jr, Lo TW, Thornalley PJ: Methylglyoxal concentration and glyoxalase activities in the human lens. Exp Eye Res. 1994 Oct;59(4):497-500. Pubmed: 7859825
- Jan CR, Chen CH, Wang SC, Kuo SY: Effect of methylglyoxal on intracellular calcium levels and viability in renal tubular cells. Cell Signal. 2005 Jul;17(7):847-55. Epub 2004 Dec 8. Pubmed: 15763427
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Kuhla B, Luth HJ, Haferburg D, Boeck K, Arendt T, Munch G: Methylglyoxal, glyoxal, and their detoxification in Alzheimer's disease. Ann N Y Acad Sci. 2005 Jun;1043:211-6. Pubmed: 16037241
- Lo TW, Selwood T, Thornalley PJ: The reaction of methylglyoxal with aminoguanidine under physiological conditions and prevention of methylglyoxal binding to plasma proteins. Biochem Pharmacol. 1994 Nov 16;48(10):1865-70. Pubmed: 7986197
- Mottaran E, Stewart SF, Rolla R, Vay D, Cipriani V, Moretti M, Vidali M, Sartori M, Rigamonti C, Day CP, Albano E: Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Radic Biol Med. 2002 Jan 1;32(1):38-45. Pubmed: 11755315
- Nemet I, Varga-Defterdarovic L, Turk Z: Preparation and quantification of methylglyoxal in human plasma using reverse-phase high-performance liquid chromatography. Clin Biochem. 2004 Oct;37(10):875-81. Pubmed: 15369718
- Riley ML, Harding JJ: The reaction of methylglyoxal with human and bovine lens proteins. Biochim Biophys Acta. 1995 Jan 25;1270(1):36-43. Pubmed: 7827133
- Schupp N, Schinzel R, Heidland A, Stopper H: Genotoxicity of advanced glycation end products: involvement of oxidative stress and of angiotensin II type 1 receptors. Ann N Y Acad Sci. 2005 Jun;1043:685-95. Pubmed: 16037294
- Seppanen P, Alhonen-Hongisto L, Janne J: Polyamine deprivation-induced enhanced uptake of methylglyoxal bis(guanylhydrazone) by tumor cells. Biochim Biophys Acta. 1981 May 5;674(2):169-77. Pubmed: 6786360
- Shamsi FA, Lin K, Sady C, Nagaraj RH: Methylglyoxal-derived modifications in lens aging and cataract formation. Invest Ophthalmol Vis Sci. 1998 Nov;39(12):2355-64. Pubmed: 9804144
- Thornalley PJ, Argirova M, Ahmed N, Mann VM, Argirov O, Dawnay A: Mass spectrometric monitoring of albumin in uremia. Kidney Int. 2000 Nov;58(5):2228-34. Pubmed: 11044246
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
- Wondrak GT, Cervantes-Laurean D, Roberts MJ, Qasem JG, Kim M, Jacobson EL, Jacobson MK: Identification of alpha-dicarbonyl scavengers for cellular protection against carbonyl stress. Biochem Pharmacol. 2002 Feb 1;63(3):361-73. Pubmed: 11853687
|
|---|