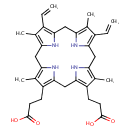
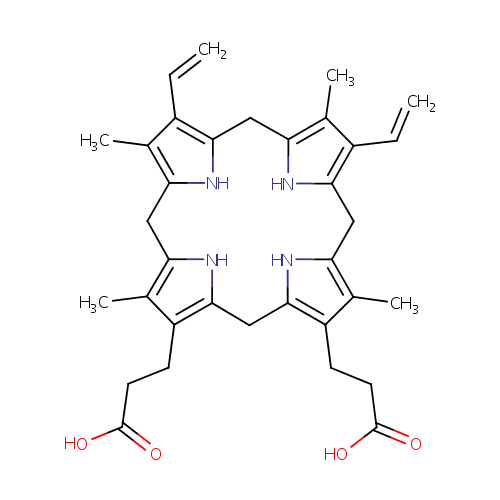
Protoporphyrinogen IX (PAMDB000254)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000254 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Protoporphyrinogen IX | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Protoporphyrinogen IX is an intermediate in heme biosynthesis. It is a porphyrinogen in which 2 pyrrole rings each have one methyl and one propionate side chain and the other two pyrrole rings each have one methyl and one vinyl side chain. 15 isomers are possible but only one, type IX, occurs naturally. Protoporphyrinogen is produced by oxidative decarboxylation of coproporphyrinogen. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C34H40N4O4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 568.7058 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 568.304955788 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | UHSGPDMIQQYNAX-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C34H40N4O4/c1-7-21-17(3)25-13-26-19(5)23(9-11-33(39)40)31(37-26)16-32-24(10-12-34(41)42)20(6)28(38-32)15-30-22(8-2)18(4)27(36-30)14-29(21)35-25/h7-8,35-38H,1-2,9-16H2,3-6H3,(H,39,40)(H,41,42) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 7412-77-3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 3-[20-(2-carboxyethyl)-10,15-diethenyl-5,9,14,19-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(20),3,5,8,10,13,15,18-octaen-4-yl]propanoic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | protoporphyrinogen | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC1=C2CC3=C(C)C(C=C)=C(CC4=C(C)C(C=C)=C(CC5=C(C)C(CCC(O)=O)=C(CC(N2)=C1CCC(O)=O)N5)N4)N3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as porphyrins. These are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Tetrapyrroles and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Porphyrins | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Porphyrins | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Membrane | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Coproporphyrin III + 2 Hydrogen ion + Oxygen <>2 Carbon dioxide +2 Water + Protoporphyrinogen IX Oxygen + Protoporphyrinogen IX >3 Water + Protoporphyrin IX 3 Fumaric acid + Protoporphyrinogen IX > Protoporphyrin IX +3 Succinic acid 2 S-Adenosylmethionine + Coproporphyrin III <>2 Carbon dioxide +2 5'-Deoxyadenosine +2 L-Methionine + Protoporphyrinogen IX Coproporphyrin III + Oxygen <> Protoporphyrinogen IX +2 Carbon dioxide +2 Water Protoporphyrinogen IX + 3 Menaquinone + 3 Menaquinone <> Protoporphyrin IX +3 Menaquinol 6 Coproporphyrinogen III + S-Adenosylmethionine > Protoporphyrinogen IX + Carbon dioxide + L-Methionine + 5'-Deoxyadenosine Protoporphyrinogen IX + Oxygen > Protoporphyrin IX + Hydrogen peroxide Coproporphyrinogen III + Oxygen + Hydrogen ion > Protoporphyrinogen IX + Carbon dioxide + Water Protoporphyrinogen IX + a menaquinone > Protoporphyrin IX + a menaquinol Protoporphyrinogen IX + 3 Menaquinone > Protoporphyrin IX +3 Menaquinol 6 S-adenosyl-L-methionine + Coproporphyrinogen III > 5'-Deoxyadenosine + L-Methionine + Carbon dioxide + Protoporphyrinogen IX Protoporphyrinogen IX + menaquinone-8 > Protoporphyrin IX + Menaquinol 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in coproporphyrinogen oxidase activity
- Specific function:
- Anaerobic transformation of coproporphyrinogen-III into protoporphyrinogen-IX
- Gene Name:
- hemN
- Locus Tag:
- PA1546
- Molecular weight:
- 52.5 kDa
Reactions
| Coproporphyrinogen-III + 2 S-adenosyl-L-methionine = protoporphyrinogen-IX + 2 CO(2) + 2 L-methionine + 2 5'-deoxyadenosine. |
- General function:
- Involved in coproporphyrinogen oxidase activity
- Specific function:
- Key enzyme in heme biosynthesis. Catalyzes the oxidative decarboxylation of propionic acid side chains of rings A and B of coproporphyrinogen III
- Gene Name:
- hemF
- Locus Tag:
- PA0024
- Molecular weight:
- 34.8 kDa
Reactions
| Coproporphyrinogen-III + O(2) + 2 H(+) = protoporphyrinogen-IX + 2 CO(2) + 2 H(2)O. |
- General function:
- Involved in coproporphyrinogen oxidase activity
- Specific function:
- Not Available
- Gene Name:
- yggW
- Locus Tag:
- PA0386
- Molecular weight:
- 42.5 kDa