|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000193 |
|---|
|
Identification |
|---|
| Name: |
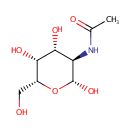
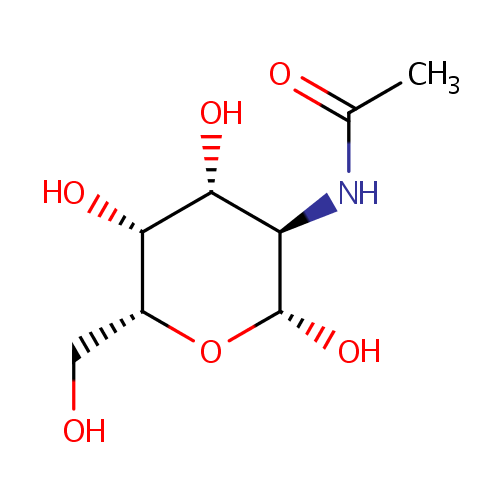
N-Acetyl-b-D-galactosamine |
|---|
| Description: | N-Acetyl-b-D-galactosamine is one of the eight essential sugars. It is important for cell communication. In galactose metabolism pathway, it is converted to N-Acetyl-b-D-galactosamine-6P by N-acetylgalactosamine-specific phosphotransferase enzyme B (EC 2.7.1.69). |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2-Deoxy-2-acetamido-b-D-galactopyranose
- 2-Deoxy-2-acetamido-b-delta-galactopyranose
- 2-Deoxy-2-acetamido-b-δ-galactopyranose
- 2-Deoxy-2-acetamido-beta-D-galactopyranose
- 2-Deoxy-2-acetamido-beta-delta-galactopyranose
- 2-Deoxy-2-acetamido-β-D-galactopyranose
- 2-Deoxy-2-acetamido-β-δ-galactopyranose
- B-D-2-Acetamido-2-deoxy-Galactopyranose
- b-delta-2-acetamido-2-Deoxy-galactopyranose
- B-N-Acetyl-D-galactosamine
- b-N-Acetyl-delta-galactosamine
- b-N-Acetyl-δ-galactosamine
- B-N-Acetylgalactosamine
- b-δ-2-acetamido-2-Deoxy-galactopyranose
- Beta-D-2-Acetamido-2-deoxy-Galactopyranose
- Beta-delta-2-Acetamido-2-deoxy-Galactopyranose
- Beta-N-Acetyl-D-galactosamine
- Beta-N-Acetyl-delta-galactosamine
- Beta-N-Acetylgalactosamine
- β-D-2-acetamido-2-Deoxy-galactopyranose
- β-N-Acetyl-D-galactosamine
- β-N-Acetyl-δ-galactosamine
- β-N-Acetylgalactosamine
- β-δ-2-acetamido-2-Deoxy-galactopyranose
|
|---|
|
Chemical Formula: |
C8H15NO6 |
|---|
| Average Molecular Weight: |
221.2078 |
|---|
| Monoisotopic Molecular
Weight: |
221.089937217 |
|---|
| InChI Key: |
OVRNDRQMDRJTHS-JAJWTYFOSA-N |
|---|
| InChI: | InChI=1S/C8H15NO6/c1-3(11)9-5-7(13)6(12)4(2-10)15-8(5)14/h4-8,10,12-14H,2H2,1H3,(H,9,11)/t4-,5-,6+,7-,8-/m1/s1 |
|---|
| CAS
number: |
14131-60-3 |
|---|
| IUPAC Name: | N-[(2R,3R,4R,5R,6R)-2,4,5-trihydroxy-6-(hydroxymethyl)oxan-3-yl]acetamide |
|---|
|
Traditional IUPAC Name: |
N-acetyl-β-D-galactosamine |
|---|
| SMILES: | CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@H](O)[C@@H]1O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organooxygen compounds |
|---|
|
Class |
Carbohydrates and carbohydrate conjugates |
|---|
| Sub Class | Aminosaccharides |
|---|
|
Direct Parent |
Acylaminosugars |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Acylaminosugar
- N-acyl-alpha-hexosamine
- Oxane
- Monosaccharide
- Acetamide
- Secondary carboxylic acid amide
- Secondary alcohol
- Polyol
- Hemiacetal
- Carboxamide group
- 1,2-diol
- Oxacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Carboxylic acid amide
- Hydrocarbon derivative
- Primary alcohol
- Organonitrogen compound
- Carbonyl group
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Barbas C, Garcia A, de Miguel L, Simo C: Evaluation of filter paper collection of urine samples for detection and measurement of organic acidurias by capillary electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci. 2002 Nov 15;780(1):73-82. Pubmed: 12383482
- Garcia A, Barbas C, Aguilar R, Castro M: Capillary electrophoresis for rapid profiling of organic acidurias. Clin Chem. 1998 Sep;44(9):1905-11. Pubmed: 9732975
- Gheri G, Bryk SG, Taddei G, Moncini D, Noci I: Sugar residues content and distribution in atrophic and hyperplastic postmenopausal human endometrium: lectin histochemistry. Histol Histopathol. 1996 Oct;11(4):861-7. Pubmed: 8930627
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Ohdoi C, Nyhan WL, Kuhara T: Chemical diagnosis of Lesch-Nyhan syndrome using gas chromatography-mass spectrometry detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Jul 15;792(1):123-30. Pubmed: 12829005
- Sediva A, Stejskal J, Bartunkova J, Smetana K Jr, Gabius HJ: Detection of alpha(beta)-N-acetyl-D-galactosamine-binding sites in kidney--relation to Henoch-Schonlein-associated IgA nephropathy. Folia Biol (Praha). 1999;45(4):147-50. Pubmed: 10732728
|
|---|
| Synthesis Reference: |
Chaplin, David; Crout, David H. G.; Bornemann, Stephen; Hutchinson, David W.; Khan, Riaz. Conversion of 2-acetamido-2-deoxy-b-D-glucopyranose (N-acetylglucosamine) into 2-acetamido-2-deoxy-b-D-galactopyranose (N-acetylgalactosamine using a biotransformat |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|