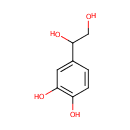
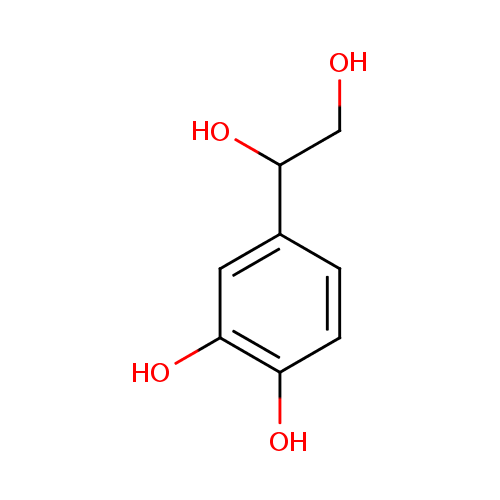
| References: |
- Baskys A, Fang L, Bayazitov I: Activation of neuroprotective pathways by metabotropic group I glutamate receptors: a potential target for drug discovery? Ann N Y Acad Sci. 2005 Aug;1053:55-73. Pubmed: 16179509
- Divers WA Jr, Wilkes MM, Babaknia A, Yen SS: Maternal smoking and elevation of catecholamines and metabolites in the amniotic fluid. Am J Obstet Gynecol. 1981 Nov 15;141(6):625-8. Pubmed: 7315891
- Divers WA, Wilkes MM, Babaknia A, Hill LM, Quilligan EJ, Yen SS: Amniotic fluid catecholamines and metabolites in intrauterine growth retardation. Am J Obstet Gynecol. 1981 Nov 15;141(6):608-10. Pubmed: 7315888
- Eisenhofer G, Brush JE, Cannon RO 3rd, Stull R, Kopin IJ, Goldstein DS: Plasma dihydroxyphenylalanine and total body and regional noradrenergic activity in humans. J Clin Endocrinol Metab. 1989 Feb;68(2):247-55. Pubmed: 2563731
- Eisenhofer G, Kopin IJ, Goldstein DS: Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004 Sep;56(3):331-49. Pubmed: 15317907
- Elsworth JD, Roth RH, Redmond DE Jr: Relative importance of 3-methoxy-4-hydroxyphenylglycol and 3,4-dihydroxyphenylglycol as norepinephrine metabolites in rat, monkey, and humans. J Neurochem. 1983 Sep;41(3):786-93. Pubmed: 6875564
- Esler MD, Lambert GW, Ferrier C, Kaye DM, Wallin BG, Kalff V, Kelly MJ, Jennings GL: Central nervous system noradrenergic control of sympathetic outflow in normotensive and hypertensive humans. Clin Exp Hypertens. 1995 Jan-Feb;17(1-2):409-23. Pubmed: 7735286
- Graham PE, Smythe GA, Edwards GA, Lazarus L: Laboratory diagnosis of phaeochromocytoma: which analytes should we measure? Ann Clin Biochem. 1993 Mar;30 ( Pt 2):129-34. Pubmed: 8466142
- Julien C, Rodriguez C, Sacquet J, Cuisinaud G, Sassard J: Liquid-chromatographic determination of free and total 3,4-dihydroxyphenylglycol and 3-methoxy-4-hydroxyphenylglycol in urine. Clin Chem. 1988 May;34(5):966-9. Pubmed: 3370800
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Loo H, Scatton B, Dennis T, Benkelfat C, Gay C, Poirier-Littre MF, Garreau M, Vanelle JM, Olie JP, Deniker P: [Study of noradrenaline metabolism in depressed patients by the determination of plasma dihydroxyphenylethylene glycol]. Encephale. 1983;9(4):297-316. Pubmed: 6671452
- Machida M, Sakaguchi A, Kamada S, Fujimoto T, Takechi S, Kakinoki S, Nomura A: Simultaneous analysis of human plasma catecholamines by high-performance liquid chromatography with a reversed-phase triacontylsilyl silica column. J Chromatogr B Analyt Technol Biomed Life Sci. 2006 Jan 18;830(2):249-54. Epub 2005 Nov 21. Pubmed: 16301006
- Nakada T, Sasagawa I, Kubota Y, Suzuki H, Ishigooka M, Watanabe M: Dihydroxyphenylglycol in pheochromocytoma: its diagnostic use for norepinephrine dominant tumor. J Urol. 1996 Jan;155(1):14-8. Pubmed: 7490813
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. Pubmed: 19212411
|
|---|