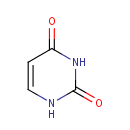
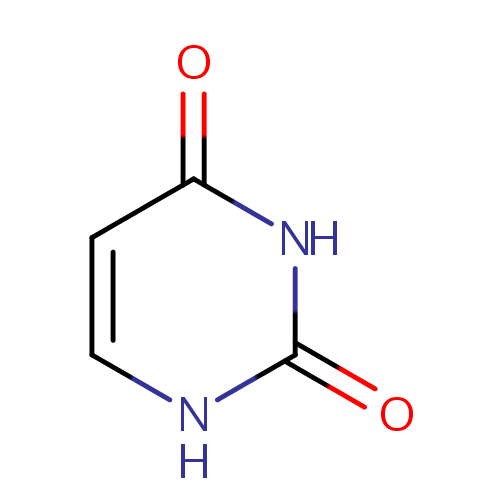
Uracil (PAMDB000126)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000126 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Uracil | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Uracil is a common naturally occurring pyrimidine found in RNA, it base pairs with adenine and is replaced by thymine in DNA. Methylation of uracil produces thymine. Uracil serves as allosteric regulator and coenzyme for many important biochemical reactions. Uracil is also involved in the biosynthesis of polysaccharides and the transportation of sugars containing aldehydes. In Pseudomonas aeruginosa, uracil catabolism is regulated by the amount of metabolically available nitrogen. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C4H4N2O2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 112.0868 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 112.027277382 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | ISAKRJDGNUQOIC-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C4H4N2O2/c7-3-1-2-5-4(8)6-3/h1-2H,(H2,5,6,7,8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 66-22-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 1,2,3,4-tetrahydropyrimidine-2,4-dione | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | uracil | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | O=C1NC=CC(=O)N1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as pyrimidones. These are compounds that contain a pyrimidine ring,which bears a ketone. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Diazines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pyrimidines and pyrimidine derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyrimidones | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 330 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Hydrogen ion + NADH + Oxygen + Uracil > NAD + Ureidoacrylate peracid Dihydrouracil + NAD <> Hydrogen ion + NADH + Uracil Water + Uridine > Ribose + Uracil Deoxyuridine + Phosphate <> Deoxyribose 1-phosphate + Uracil Cytosine + Hydrogen ion + Water > Ammonium + Uracil Phosphoribosyl pyrophosphate + Uracil <> Pyrophosphate + Uridine 5'-monophosphate Phosphate + Uridine <> Ribose-1-phosphate + Uracil Cytosine + Water <> Uracil + Ammonia Uridine + Phosphate <> Uracil + alpha-D-Ribose 1-phosphate + Ribose-1-phosphate Uracil + FMNH + Oxygen <> Ureidoacrylate peracid + Flavin Mononucleotide Deoxyuridine + Phosphate <> deoxyribose-1-phosphate + Uracil D-Ribose-5-phosphate + Uracil <> Water + Pseudouridine 5'-phosphate Uracil + Oxygen + FMNH > Hydrogen ion + Ureidoacrylate peracid + Flavin Mononucleotide Uridine + Water > D-ribose + Uracil Dihydrouracil + NAD > Uracil + NADH Uracil + FMNH(2) + Oxygen > Ureidoacrylate peracid + Flavin Mononucleotide + Water Uridine + Inorganic phosphate > Uracil + Ribose-1-phosphate More...Dihydrouracil + NAD + Dihydrothymine <> Uracil + NADH + Hydrogen ion + Thymine Uracil + FMNH + Oxygen + Thymine <> Ureidoacrylate peracid + Flavin Mononucleotide + (Z)-2-Methyl-ureidoacrylate peracid Pseudouridine 5'-phosphate + Water > Uracil + D-ribofuranose 5-phosphate + D-ribofuranose 5-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Burckhalter, J. H.; Scarborough, Homer C. The synthesis of uracils as anticonvulsants. Journal of the American Pharmaceutical Association (1912-1977) (1955), 44 545-50. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in nucleoside metabolic process
- Specific function:
- Catalyzes the conversion of uracil and 5-phospho-alpha- D-ribose 1-diphosphate (PRPP) to UMP and diphosphate
- Gene Name:
- upp
- Locus Tag:
- PA4646
- Molecular weight:
- 22.9 kDa
Reactions
| UMP + diphosphate = uracil + 5-phospho-alpha-D-ribose 1-diphosphate. |
- General function:
- Involved in hydrolase activity, acting on carbon-nitrogen (but not peptide) bonds
- Specific function:
- Cytosine + H(2)O = uracil + NH(3)
- Gene Name:
- codA
- Locus Tag:
- PA0437
- Molecular weight:
- 47.1 kDa
Reactions
| Cytosine + H(2)O = uracil + NH(3). |
Transporters
- General function:
- Involved in transporter activity
- Specific function:
- Transport of uracil in the cell
- Gene Name:
- uraA
- Locus Tag:
- PA4647
- Molecular weight:
- 44.2 kDa