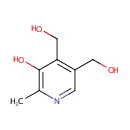
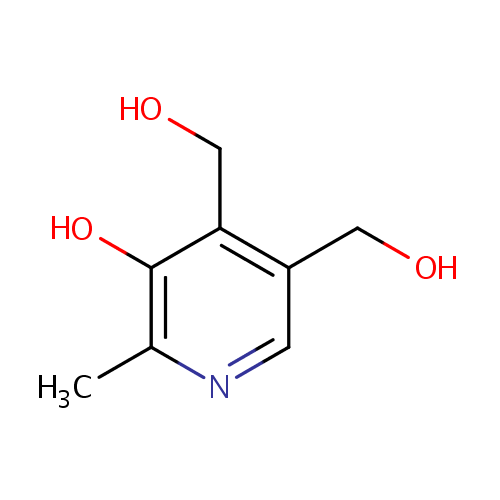
| References: |
- Amoroso A, Pirulli D, Florian F, Puzzer D, Boniotto M, Crovella S, Zezlina S, Spano A, Mazzola G, Savoldi S, Ferrettini C, Berutti S, Petrarulo M, Marangella M: AGXT gene mutations and their influence on clinical heterogeneity of type 1 primary hyperoxaluria. J Am Soc Nephrol. 2001 Oct;12(10):2072-9. Pubmed: 11562405
- Chen S, Ito M, Saijo T, Naito E, Kuroda Y: Molecular genetic analysis of pyridoxine-nonresponsive homocystinuric siblings with different blood methionine levels during the neonatal period. J Med Invest. 1999 Aug;46(3-4):186-91. Pubmed: 10687314
- Chiang EP, Selhub J, Bagley PJ, Dallal G, Roubenoff R: Pyridoxine supplementation corrects vitamin B6 deficiency but does not improve inflammation in patients with rheumatoid arthritis. Arthritis Res Ther. 2005;7(6):R1404-11. Epub 2005 Oct 14. Pubmed: 16277693
- Esteve-Romero J, Capella-Peiro ME, Monferrer-Pons L, Gil-Agusti M: Micellar liquid chromatography in clinical chemistry: application to the monitorization of B6 vitamins. Clin Chim Acta. 2004 Oct;348(1-2):69-77. Pubmed: 15369738
- Henderson JM, Codner MA, Hollins B, Kutner MH, Merrill AH: The fasting B6 vitamer profile and response to a pyridoxine load in normal and cirrhotic subjects. Hepatology. 1986 May-Jun;6(3):464-71. Pubmed: 3710434
- Ishii, N., Nakahigashi, K., Baba, T., Robert, M., Soga, T., Kanai, A., Hirasawa, T., Naba, M., Hirai, K., Hoque, A., Ho, P. Y., Kakazu, Y., Sugawara, K., Igarashi, S., Harada, S., Masuda, T., Sugiyama, N., Togashi, T., Hasegawa, M., Takai, Y., Yugi, K., Arakawa, K., Iwata, N., Toya, Y., Nakayama, Y., Nishioka, T., Shimizu, K., Mori, H., Tomita, M. (2007). "Multiple high-throughput analyses monitor the response of E. coli to perturbations." Science 316:593-597. Pubmed: 17379776
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Kidd GS, Dimond R, Kark JA, Whorton N, Vigersky RA: The effects of pyridoxine on pituitary hormone secretion in amenorrhea-galactorrhea syndromes. J Clin Endocrinol Metab. 1982 Apr;54(4):872-5. Pubmed: 6801073
- Manyam BV, Ferraro TN, Hare TA: Isoniazid-induced alteration of CSF neurotransmitter amino acids in Huntington's disease. Brain Res. 1987 Apr 7;408(1-2):125-30. Pubmed: 2885064
- McCully KS: Homocysteine, vitamins, and prevention of vascular disease. Mil Med. 2004 Apr;169(4):325-9. Pubmed: 15132238
- Pirulli D, Marangella M, Amoroso A: Primary hyperoxaluria: genotype-phenotype correlation. J Nephrol. 2003 Mar-Apr;16(2):297-309. Pubmed: 12768081
- Plecko B, Stockler-Ipsiroglu S, Paschke E, Erwa W, Struys EA, Jakobs C: Pipecolic acid elevation in plasma and cerebrospinal fluid of two patients with pyridoxine-dependent epilepsy. Ann Neurol. 2000 Jul;48(1):121-5. Pubmed: 10894227
- Temesvari P, Szilagyi I, Eck E, Boda D: Effects of an antenatal load of pyridoxine (vitamin B6) on the blood oxygen affinity and prolactin levels in newborn infants and their mothers. Acta Paediatr Scand. 1983 Jul;72(4):525-9. Pubmed: 6624427
- Tolis G, Laliberte R, Guyda H, Naftolin F: Ineffectiveness of pyridoxine (B6) to alter secretion of growth hormone and prolactin and absence of therapeutic effects on galactorrhea-amenorrhea syndromes. J Clin Endocrinol Metab. 1977 Jun;44(6):1197-9. Pubmed: 559690
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Wasilewska A, Narkiewicz M, Rutkowski B, Lysiak-Szydlowska W: Is there any relationship between lipids and vitamin B levels in persons with elevated risk of atherosclerosis? Med Sci Monit. 2003 Mar;9(3):CR147-51. Pubmed: 12640345
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|