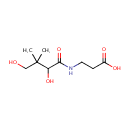
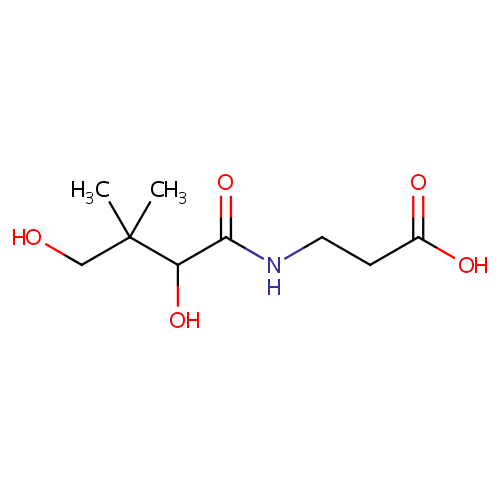
Pantothenic acid (PAMDB000085)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000085 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Pantothenic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Pantothenic acid, also called vitamin B5, is a water-soluble vitamin required to sustain life. Pantothenic acid is needed to form coenzyme-A (CoA), and is thus critical in the metabolism and synthesis of carbohydrates, proteins, and fats. Its name is derived from the Greek pantothen meaning "from everywhere" and small quantities of pantothenic acid are found in nearly every food, with high amounts in whole grain cereals, legumes, eggs, meat, and royal jelly. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C9H17NO5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 219.235 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 219.110672659 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | GHOKWGTUZJEAQD-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C9H17NO5/c1-9(2,5-11)7(14)8(15)10-4-3-6(12)13/h7,11,14H,3-5H2,1-2H3,(H,10,15)(H,12,13) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 79-83-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 3-(2,4-dihydroxy-3,3-dimethylbutanamido)propanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | DL-pantothenic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC(C)(CO)C(O)C(=O)NCCC(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as beta amino acids and derivatives. These are amino acids having a (-NH2) group attached to the beta carbon atom. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carboxylic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Amino acids, peptides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Beta amino acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | < 25 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | beta-Alanine + Adenosine triphosphate + (R)-Pantoate <> Adenosine monophosphate + Hydrogen ion + Pantothenic acid + Pyrophosphate Adenosine triphosphate + Pantothenic acid <> D-4'-Phosphopantothenate + ADP + Hydrogen ion Adenosine triphosphate + (R)-Pantoate + beta-Alanine <> Adenosine monophosphate + Pyrophosphate + Pantothenic acid Adenosine triphosphate + Pantothenic acid <> ADP + D-4'-Phosphopantothenate Pantetheine + Water + Pantetheine > Pantothenic acid + Cysteamine + Pantothenic acid Pantothenic acid + Adenosine triphosphate + Pantothenic acid > D-4'-Phosphopantothenate + Adenosine diphosphate + D-4'-Phosphopantothenate + ADP beta-Alanine + Adenosine triphosphate + (R)-pantoate + (R)-Pantoate > Adenosine monophosphate + Pyrophosphate + Hydrogen ion + Pantothenic acid + Pantothenic acid Pantothenic acid + Adenosine triphosphate + Pantothenic acid > Adenosine diphosphate + Hydrogen ion + D-4'-Phosphopantothenate + ADP + D-4'-Phosphopantothenate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Kataoka, Michihiko; Shimizu, Sakayu; Doi, Yukiko; Yamada, Hideaki. Stereospecific reduction of ethyl 2'-ketopantothenate to ethyl D-(+)-pantothenate with microbial cells as a catalyst. Applied and Environmental Microbiology (1990), 56(11), 3595-7. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in catalytic activity
- Specific function:
- Catalyzes the condensation of pantoate with beta-alanine in an ATP-dependent reaction via a pantoyl-adenylate intermediate
- Gene Name:
- panC
- Locus Tag:
- PA4730
- Molecular weight:
- 30.8 kDa
Reactions
| ATP + (R)-pantoate + beta-alanine = AMP + diphosphate + (R)-pantothenate. |