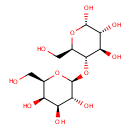
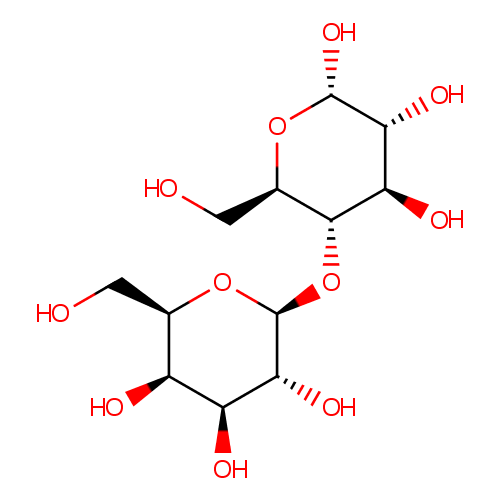
| References: |
- Bondesson E, Bengtsson T, Borgstrom L, Nilsson LE, Norrgren K, Olsson B, Svensson M, Wollmer P: Dose delivery late in the breath can increase dry powder aerosol penetration into the lungs. J Aerosol Med. 2005 Spring;18(1):23-33. Pubmed: 15741771
- Delaveau P: [Milk lactose. Hypothesis on its biological importance]. Ann Pharm Fr. 2003;61(5):340-2. Pubmed: 13130292
- Dimopoulos MA, Anagnostopoulos A: Thalidomide in relapsed/refractory multiple myeloma: pivotal trials conducted outside the United States. Semin Hematol. 2003 Oct;40(4 Suppl 4):8-16. Pubmed: 15015891
- Gunther S, Patterson RE, Kristal AR, Stratton KL, White E: Demographic and health-related correlates of herbal and specialty supplement use. J Am Diet Assoc. 2004 Jan;104(1):27-34. Pubmed: 14702580
- Johnson JD, Simoons FJ, Hurwitz R, Grange A, Mitchell CH, Sinatra FR, Sunshine P, Robertson WV, Bennett PH, Kretchmer N: Lactose malabsorption among the Pima indians of Arizona. Gastroenterology. 1977 Dec;73(6):1299-304. Pubmed: 578795
- Jung SK, Fujimoto D: A novel beta-galactoside-binding lectin in adult rat kidney. J Biochem (Tokyo). 1994 Sep;116(3):547-53. Pubmed: 7852273
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Kim KI, Lee WS, Benevenga NJ: Feeding diets containing high levels of milk products or cellulose decrease urease activity and ammonia production in rat intestine. J Nutr. 1998 Jul;128(7):1186-91. Pubmed: 9649604
- Lustenberger RW: [A 23-year old patient with chronic diarrhea. Celiac disease and lactose intolerance] Schweiz Rundsch Med Prax. 2005 Feb 2;94(5):163-4. Pubmed: 15745382
- Mitchell JD, Brand J, Halbisch J: Weight-gain inhibition by lactose in Australian Aboriginal children. A controlled trial of normal and lactose hydrolysed milk. Lancet. 1977 Mar 5;1(8010):500-2. Pubmed: 65606
- Muthusamy A, Erickson DR, Sheykhnazari M, Bhavanandan VP: Enhanced binding of modified pentosan polysulfate and heparin to bladder--a strategy for improved treatment of interstitial cystitis. Urology. 2006 Jan;67(1):209-13. Pubmed: 16413377
- Oozeer R, Furet JP, Goupil-Feuillerat N, Anba J, Mengaud J, Corthier G: Differential activities of four Lactobacillus casei promoters during bacterial transit through the gastrointestinal tracts of human-microbiota-associated mice. Appl Environ Microbiol. 2005 Mar;71(3):1356-63. Pubmed: 15746338
- Rana SV, Bhasin DK, Vinayak VK: Lactose hydrogen breath test in Giardia lamblia-positive patients. Dig Dis Sci. 2005 Feb;50(2):259-61. Pubmed: 15745082
- Roberson CM: Lactose intolerance. Ala Nurse. 2004 Dec-2005 Feb;31(4):23-4; quiz 24. Pubmed: 15662762
- Sharma A, DiCioccio RA, Allen HJ: Identification and synthesis of a novel 15 kDa beta-galactoside-binding lectin in human leukocytes. Glycobiology. 1992 Aug;2(4):285-92. Pubmed: 1421750
- Soupene E, van Heeswijk WC, Plumbridge J, Stewart V, Bertenthal D, Lee H, Prasad G, Paliy O, Charernnoppakul P, Kustu S: Physiological studies of Escherichia coli strain MG1655: growth defects and apparent cross-regulation of gene expression. J Bacteriol. 2003 Sep;185(18):5611-26. Pubmed: 12949114
- Swagerty DL Jr, Walling AD, Klein RM: Lactose intolerance. Am Fam Physician. 2002 May 1;65(9):1845-50. Pubmed: 12018807
- Szilagyi A: Review article: lactose--a potential prebiotic. Aliment Pharmacol Ther. 2002 Sep;16(9):1591-602. Pubmed: 12197838
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Vilotte JL: Lowering the milk lactose content in vivo: potential interests, strategies and physiological consequences. Reprod Nutr Dev. 2002 Mar-Apr;42(2):127-32. Pubmed: 12216958
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
- Yeoh E, Horowitz M, Russo A, Muecke T, Robb T, Chatterton B: The effects of abdominal irradiation for seminoma of the testis on gastrointestinal function. J Gastroenterol Hepatol. 1995 Mar-Apr;10(2):125-30. Pubmed: 7787155
|
|---|