Search Results for compounds
Searching compounds for
returned 4373 results.
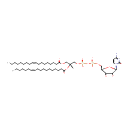
CDP-DG(18:1(9Z)/18:1(11Z)) (PAMDB000737)
IUPAC:
[({[(2R,3R,5R)-5-(4-amino-2-oxo-1,2-dihydropyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy][(2R)-2-[(11Z)-octadec-11-enoyloxy]-3-[(9Z)-octadec-9-enoyloxy]propoxy]phosphinic acid
CAS: Not Available
Description: CDP-DG(18:1(9Z)/18:1(11Z)) belongs to the family of CDP-diacylglycerols. It is a glycerophospholipid containing a diacylglycerol, with a cytidine diphosphate attached to the oxygen O1 or O2 of the glycerol part. As is the case with diacylglycerols, phosphatidylserines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. CDP-DG(18:1(9Z)/18:1(11Z)), in particular, consists of one 9Z-octadecenoyl chain to C-1 atom, and one 11Z-octadecenoyl to the C-2 atom. In Pseudomonas aeruginosa glycerophospholipid metabolism, The biosynthesis of CDP-diacylglycerol (CDP-DG) involves condensation of phosphatidic acid (PA) and cytidine triphosphate, with elimination of pyrophosphate, catalysed by the enzyme CDP-diacylglycerol synthase. The resulting CDP-diacylglycerol can be utilized immediately for the synthesis of phosphatidylglycerol (PG), and thence cardiolipin (CL), and of phosphatidylinositol (PI). CDP-DG(18:1(9Z)/18:1(11Z)) is also a substrate of CDP-diacylglycerol pyrophosphatase. It is involved in CDP-diacylglycerol degradation pathway.
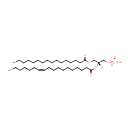
PA(16:0/18:1(11Z)) (PAMDB000743)
IUPAC:
[(2R)-3-(hexadecanoyloxy)-2-[(11Z)-octadec-11-enoyloxy]propoxy]phosphonic acid
CAS: Not Available
Description: PA(16:0/18:1(11Z)) is a phosphatidic acid. It is a glycerophospholipid in which a phosphate moiety occupies a glycerol substitution site. As is the case with diacylglycerols, phosphatidic acids can have many different combinations of fatty acids of varying lengths and saturation attached at the C-1 and C-2 positions. Fatty acids containing 16, 18 and 20 carbons are the most common. PA(16:0/18:1(11Z)), in particular, consists of one chain of palmitic acid at the C-1 position and one chain of vaccenic acid at the C-2 position. Phosphatidic acids are quite rare but are extremely important as intermediates in the biosynthesis of triacylglycerols and phospholipids. PAs are biologically active lipids that can stimulate a large range of responses in many different cell types. Diacylglycerols (DAGs) can be converted to PAs by DAG kinases. Phospholipase Ds (PLDs), which catalyze the conversion of glycerolphospholipids, particularly phosphatidylcholine, to PAs and the conversion of N-arachidonoyl-phosphatidylethanolamine (NAPE) to anandamide and PAs are activated by several inflammatory mediators including bradykinin, ATP and glutamate. PAs activate downstream signaling pathways such as PKCs and mitogen-activated protein kinases (MAPKs). Circumstantial evidence that PAs are converted to DAGs. (PMID: 12618218, 16185776).
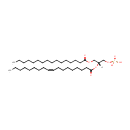
PA(16:0/18:1(9Z)) (PAMDB000744)
IUPAC:
[(2R)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propoxy]phosphonic acid
CAS: Not Available
Description: PA(16:0/18:1(9Z)) is a phosphatidic acid. It is a glycerophospholipid in which a phosphate moiety occupies a glycerol substitution site. As is the case with diacylglycerols, phosphatidic acids can have many different combinations of fatty acids of varying lengths and saturation attached at the C-1 and C-2 positions. Fatty acids containing 16, 18 and 20 carbons are the most common. PA(16:0/18:1(9Z)), in particular, consists of one chain of palmitic acid at the C-1 position and one chain of oleic acid at the C-2 position. Phosphatidic acids are quite rare but are extremely important as intermediates in the biosynthesis of triacylglycerols and phospholipids. PAs are biologically active lipids that can stimulate a large range of responses in many different cell types. Diacylglycerols (DAGs) can be converted to PAs by DAG kinases. Phospholipase Ds (PLDs), which catalyze the conversion of glycerolphospholipids, particularly phosphatidylcholine, to PAs and the conversion of N-arachidonoyl-phosphatidylethanolamine (NAPE) to anandamide and PAs are activated by several inflammatory mediators including bradykinin, ATP and glutamate. PAs activate downstream signaling pathways such as PKCs and mitogen-activated protein kinases (MAPKs). Circumstantial evidence that PAs are converted to DAGs. (PMID: 12618218, 16185776).
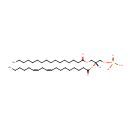
PA(16:0/18:2(9Z,12Z)) (PAMDB000745)
IUPAC:
[(2R)-3-(hexadecanoyloxy)-2-[(9Z,12Z)-octadeca-9,12-dienoyloxy]propoxy]phosphonic acid
CAS: Not Available
Description: PA(16:0/18:2(9Z,12Z)) is a phosphatidic acid. It is a glycerophospholipid in which a phosphate moiety occupies a glycerol substitution site. As is the case with diacylglycerols, phosphatidic acids can have many different combinations of fatty acids of varying lengths and saturation attached at the C-1 and C-2 positions. Fatty acids containing 16, 18 and 20 carbons are the most common. PA(16:0/18:2(9Z,12Z)), in particular, consists of one chain of palmitic acid at the C-1 position and one chain of linoleic acid at the C-2 position. Phosphatidic acids are quite rare but are extremely important as intermediates in the biosynthesis of triacylglycerols and phospholipids. PAs are biologically active lipids that can stimulate a large range of responses in many different cell types. Diacylglycerols (DAGs) can be converted to PAs by DAG kinases. Phospholipase Ds (PLDs), which catalyze the conversion of glycerolphospholipids, particularly phosphatidylcholine, to PAs and the conversion of N-arachidonoyl-phosphatidylethanolamine (NAPE) to anandamide and PAs are activated by several inflammatory mediators including bradykinin, ATP and glutamate. PAs activate downstream signaling pathways such as PKCs and mitogen-activated protein kinases (MAPKs). Circumstantial evidence that PAs are converted to DAGs. (PMID: 12618218, 16185776).
PA(18:0/18:2(9Z,12Z)) (PAMDB000746)
IUPAC:
[(2R)-2-[(9Z,12Z)-octadeca-9,12-dienoyloxy]-3-(octadecanoyloxy)propoxy]phosphonic acid
CAS: Not Available
Description: PA(18:0/18:2(9Z,12Z)) is a phosphatidic acid. It is a glycerophospholipid in which a phosphate moiety occupies a glycerol substitution site. As is the case with diacylglycerols, phosphatidic acids can have many different combinations of fatty acids of varying lengths and saturation attached at the C-1 and C-2 positions. Fatty acids containing 16, 18 and 20 carbons are the most common. PA(18:0/18:2(9Z,12Z)), in particular, consists of one chain of stearic acid at the C-1 position and one chain of linoleic acid at the C-2 position. Phosphatidic acids are quite rare but are extremely important as intermediates in the biosynthesis of triacylglycerols and phospholipids. PAs are biologically active lipids that can stimulate a large range of responses in many different cell types. Diacylglycerols (DAGs) can be converted to PAs by DAG kinases. Phospholipase Ds (PLDs), which catalyze the conversion of glycerolphospholipids, particularly phosphatidylcholine, to PAs and the conversion of N-arachidonoyl-phosphatidylethanolamine (NAPE) to anandamide and PAs are activated by several inflammatory mediators including bradykinin, ATP and glutamate. PAs activate downstream signaling pathways such as PKCs and mitogen-activated protein kinases (MAPKs). Circumstantial evidence that PAs are converted to DAGs. (PMID: 12618218, 16185776).
PA(18:1(11Z)/18:1(11Z)) (PAMDB000747)
IUPAC:
[(2R)-2,3-bis[(11Z)-octadec-11-enoyloxy]propoxy]phosphonic acid
CAS: Not Available
Description: PA(18:1(11Z)/18:1(11Z)) is a phosphatidic acid. It is a glycerophospholipid in which a phosphate moiety occupies a glycerol substitution site. As is the case with diacylglycerols, phosphatidic acids can have many different combinations of fatty acids of varying lengths and saturation attached at the C-1 and C-2 positions. Fatty acids containing 16, 18 and 20 carbons are the most common. PA(18:1(11Z)/18:1(11Z)), in particular, consists of one chain of vaccenic acid at the C-1 position and one chain of vaccenic acid at the C-2 position. Phosphatidic acids are quite rare but are extremely important as intermediates in the biosynthesis of triacylglycerols and phospholipids. PAs are biologically active lipids that can stimulate a large range of responses in many different cell types. Diacylglycerols (DAGs) can be converted to PAs by DAG kinases and indirect evidence supports the notion that PAs alter the excitability of neurons. Phospholipase Ds (PLDs), which catalyze the conversion of glycerolphospholipids, particularly phosphatidylcholine, to PAs and the conversion of N-arachidonoyl-phosphatidylethanolamine (NAPE) to anandamide and PAs are activated by several inflammatory mediators including bradykinin, ATP and glutamate. PAs activate downstream signaling pathways such as PKCs and mitogen-activated protein kinases (MAPKs). Circumstantial evidence that PAs are converted to DAGs. (PMID: 12618218, 16185776).
PA(18:1(11Z)/18:1(9Z)) (PAMDB000748)
IUPAC:
[(2R)-3-[(11Z)-octadec-11-enoyloxy]-2-[(9Z)-octadec-9-enoyloxy]propoxy]phosphonic acid
CAS: Not Available
Description: PA(18:1(11Z)/18:1(9Z)) is a phosphatidic acid. It is a glycerophospholipid in which a phosphate moiety occupies a glycerol substitution site. As is the case with diacylglycerols, phosphatidic acids can have many different combinations of fatty acids of varying lengths and saturation attached at the C-1 and C-2 positions. Fatty acids containing 16, 18 and 20 carbons are the most common. PA(18:1(11Z)/18:1(9Z)), in particular, consists of one chain of vaccenic acid at the C-1 position and one chain of oleic acid at the C-2 position. Phosphatidic acids are quite rare but are extremely important as intermediates in the biosynthesis of triacylglycerols and phospholipids. PAs are biologically active lipids that can stimulate a large range of responses in many different cell types. Diacylglycerols (DAGs) can be converted to PAs by DAG kinases. Phospholipase Ds (PLDs), which catalyze the conversion of glycerolphospholipids, particularly phosphatidylcholine, to PAs and the conversion of N-arachidonoyl-phosphatidylethanolamine (NAPE) to anandamide and PAs are activated by several inflammatory mediators including bradykinin, ATP and glutamate. PAs activate downstream signaling pathways such as PKCs and mitogen-activated protein kinases (MAPKs). Circumstantial evidence that PAs are converted to DAGs. (PMID: 12618218, 16185776).
PA(18:1(9Z)/18:1(11Z)) (PAMDB000749)
IUPAC:
[(2R)-2-[(11Z)-octadec-11-enoyloxy]-3-[(9Z)-octadec-9-enoyloxy]propoxy]phosphonic acid
CAS: Not Available
Description: PA(18:1(9Z)/18:1(11Z)) is a phosphatidic acid. It is a glycerophospholipid in which a phosphate moiety occupies a glycerol substitution site. As is the case with diacylglycerols, phosphatidic acids can have many different combinations of fatty acids of varying lengths and saturation attached at the C-1 and C-2 positions. Fatty acids containing 16, 18 and 20 carbons are the most common. PA(18:1(9Z)/18:1(11Z)), in particular, consists of one chain of oleic acid at the C-1 position and one chain of vaccenic acid at the C-2 position. Phosphatidic acids are quite rare but are extremely important as intermediates in the biosynthesis of triacylglycerols and phospholipids. PAs are biologically active lipids that can stimulate a large range of responses in many different cell types. Diacylglycerols (DAGs) can be converted to PAs by DAG kinases. Phospholipase Ds (PLDs), which catalyze the conversion of glycerolphospholipids, particularly phosphatidylcholine, to PAs and the conversion of N-arachidonoyl-phosphatidylethanolamine (NAPE) to anandamide and PAs are activated by several inflammatory mediators including bradykinin, ATP and glutamate. PAs activate downstream signaling pathways such as PKCs and mitogen-activated protein kinases (MAPKs). Circumstantial evidence that PAs are converted to DAGs. (PMID: 12618218, 16185776).
PA(18:1(9Z)/18:1(9Z)) (PAMDB000750)
IUPAC:
[(2R)-2,3-bis[(9Z)-octadec-9-enoyloxy]propoxy]phosphonic acid
CAS: 14268-17-8
Description: PA(18:1(9Z)/18:1(9Z)) is a phosphatidic acid. It is a glycerophospholipid in which a phosphate moiety occupies a glycerol substitution site. As is the case with diacylglycerols, phosphatidic acids can have many different combinations of fatty acids of varying lengths and saturation attached at the C-1 and C-2 positions. Fatty acids containing 16, 18 and 20 carbons are the most common. PA(18:1(9Z)/18:1(9Z)), in particular, consists of one chain of oleic acid at the C-1 position and one chain of oleic acid at the C-2 position. Phosphatidic acids are quite rare but are extremely important as intermediates in the biosynthesis of triacylglycerols and phospholipids. PAs are biologically active lipids that can stimulate a large range of responses in many different cell types. Diacylglycerols (DAGs) can be converted to PAs by DAG kinases. Phospholipase Ds (PLDs), which catalyze the conversion of glycerolphospholipids, particularly phosphatidylcholine, to PAs and the conversion of N-arachidonoyl-phosphatidylethanolamine (NAPE) to anandamide and PAs are activated by several inflammatory mediators including bradykinin, ATP and glutamate. PAs activate downstream signaling pathways such as PKCs and mitogen-activated protein kinases (MAPKs). Circumstantial evidence that PAs are converted to DAGs. (PMID: 12618218, 16185776).
PE(14:0/14:0) (PAMDB000751)
IUPAC:
(2-aminoethoxy)[(2R)-2,3-bis(tetradecanoyloxy)propoxy]phosphinic acid
CAS: Not Available
Description: PE(14:0/14:0) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PE(14:0/14:0), in particular, consists of two tetradecanoyl chains at positions C-1 and C-2. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.