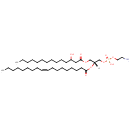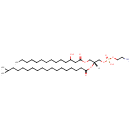
Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 2031 - 2040 of
4373 in total
PE(14:0(3-OH)/15:0cyclo) (PAMDB003872)
IUPAC:
(2-aminoethoxy)[(2R)-2-{[8-(2-butylcyclopropyl)octanoyl]oxy}-3-[(3-hydroxytetradecanoyl)oxy]propoxy]phosphinic acid
CAS: Not Available
Description: PE(14:0(3-OH)/15:0cyclo) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. PE(14:0(3-OH)/15:0cyclo), in particular, consists of one 3-hydroxytetradecanoyl chain to the C-1 atom, and one cis-9,10-Methylenetetradecanoic acid to the C-2 atom. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.
PE(14:0(3-OH)/16:0) (PAMDB003873)
IUPAC:
(2-aminoethoxy)[(2R)-2-(hexadecanoyloxy)-3-[(3-hydroxytetradecanoyl)oxy]propoxy]phosphinic acid
CAS: Not Available
Description: PE(14:0(3-OH)/16:0) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. PE(14:0(3-OH)/16:0), in particular, consists of one 3-hydroxytetradecanoyl chain to the C-1 atom, and one hexadecanoyl to the C-2 atom. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.
PE(14:0(3-OH)/16:1(9Z)) (PAMDB003874)
IUPAC:
(2-aminoethoxy)[(2R)-2-[(9Z)-hexadec-9-enoyloxy]-3-[(3-hydroxytetradecanoyl)oxy]propoxy]phosphinic acid
CAS: Not Available
Description: PE(14:0(3-OH)/16:1(9Z)) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. PE(14:0(3-OH)/16:1(9Z)), in particular, consists of one 3-hydroxytetradecanoyl chain to the C-1 atom, and one 9Z-hexadecenoyl to the C-2 atom. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.
PE(14:0(3-OH)/17:0cycw7c) (PAMDB003875)
IUPAC:
(2-aminoethoxy)[(2R)-2-{[8-(2-hexylcyclopropyl)octanoyl]oxy}-3-[(3-hydroxytetradecanoyl)oxy]propoxy]phosphinic acid
CAS: Not Available
Description: PE(14:0(3-OH)/17:0cycw7c) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. PE(14:0(3-OH)/17:0cycw7c), in particular, consists of one 3-hydroxytetradecanoyl chain to the C-1 atom, and one heptadec-9-10-cyclo-anoyl to the C-2 atom. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.
PE(14:0(3-OH)/18:1(9Z)) (PAMDB003876)
IUPAC:
(2-aminoethoxy)[(2R)-3-[(3-hydroxytetradecanoyl)oxy]-2-[(9Z)-octadec-9-enoyloxy]propoxy]phosphinic acid
CAS: Not Available
Description: PE(14:0(3-OH)/18:1(9Z)) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. PE(14:0(3-OH)/18:1(9Z)), in particular, consists of one 3-hydroxytetradecanoyl chain to the C-1 atom, and one 9Z-octadecenoyl to the C-2 atom. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.
PE(14:0(3-OH)/19:0cycv8c) (PAMDB003877)
IUPAC:
(2-aminoethoxy)[(2R)-2-{[10-(2-hexylcyclopropyl)decanoyl]oxy}-3-[(3-hydroxytetradecanoyl)oxy]propoxy]phosphinic acid
CAS: Not Available
Description: PE(14:0(3-OH)/19:0cycv8c) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. PE(14:0(3-OH)/19:0cycv8c), in particular, consists of one 3-hydroxytetradecanoyl chain to the C-1 atom, and one heptadec-11-12-cyclo-anoyl to the C-2 atom. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.
PE(14:0(3-OH)/19:iso) (PAMDB003878)
IUPAC:
(2-aminoethoxy)[(2R)-3-[(3-hydroxytetradecanoyl)oxy]-2-[(17-methyloctadecanoyl)oxy]propoxy]phosphinic acid
CAS: Not Available
Description: PE(14:0(3-OH)/19:iso) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. PE(14:0(3-OH)/19:iso), in particular, consists of one 3-hydroxytetradecanoyl chain to the C-1 atom, and one 17-methylocatdecanoyl to the C-2 atom. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.
PE(14:0/10:0(3-OH)) (PAMDB003879)
IUPAC:
(2-aminoethoxy)[(2R)-2-[(3-hydroxydecanoyl)oxy]-3-(tetradecanoyloxy)propoxy]phosphinic acid
CAS: Not Available
Description: PE(14:0/10:0(3-OH)) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. PE(14:0/10:0(3-OH)), in particular, consists of one tetradecanoyl chain to the C-1 atom, and one 3-hydroxydecanoyl to the C-2 atom. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.
PE(14:0/12:0(3-OH)) (PAMDB003880)
IUPAC:
(2-aminoethoxy)[(2R)-2-[(3-hydroxydodecanoyl)oxy]-3-(tetradecanoyloxy)propoxy]phosphinic acid
CAS: Not Available
Description: PE(14:0/12:0(3-OH)) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. PE(14:0/12:0(3-OH)), in particular, consists of one tetradecanoyl chain to the C-1 atom, and one 3-hydroxydodecanoyl to the C-2 atom. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.
PE(14:0/14:0(3-OH)) (PAMDB003881)
IUPAC:
(2-aminoethoxy)[(2R)-2-[(3-hydroxytetradecanoyl)oxy]-3-(tetradecanoyloxy)propoxy]phosphinic acid
CAS: Not Available
Description: PE(14:0/14:0(3-OH)) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. PE(14:0/14:0(3-OH)), in particular, consists of one tetradecanoyl chain to the C-1 atom, and one 3-hydroxytetradecanoyl to the C-2 atom. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.