|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120670 |
|---|
|
Identification |
|---|
| Name: |
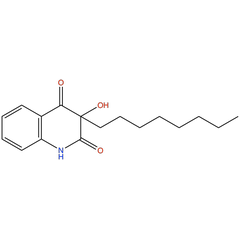
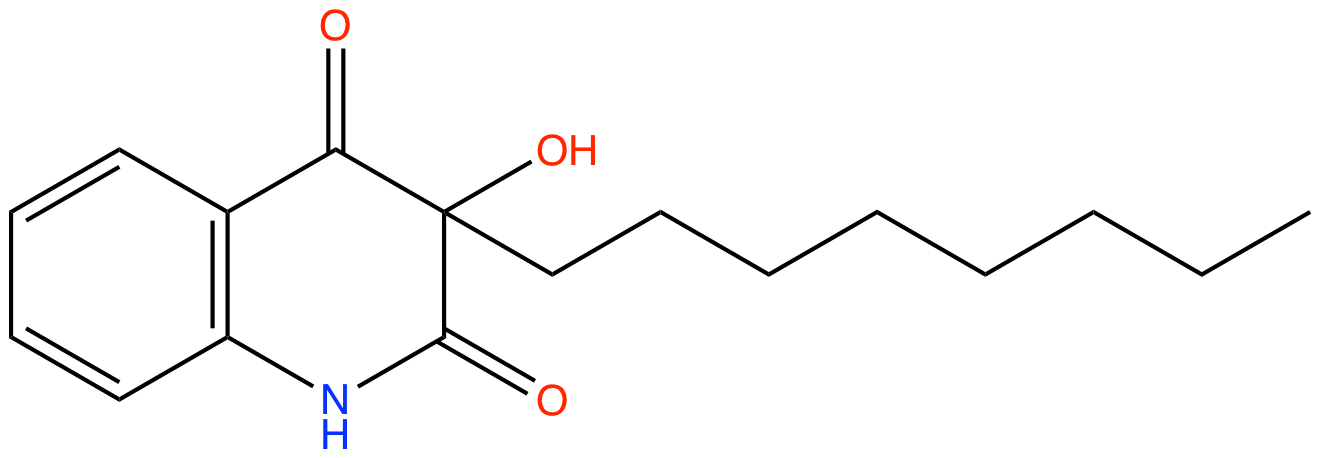
3-octyl-2,3-hydroxy-4-quinolones |
|---|
| Description: | Pseudomonas aeruginosa alkyl-quinolones (AQs) are small molecule metabolites produced by Pseudomonas aeruginosa . Pseudomonas aeruginosa produces over 55 AQ species, many of which have unknown biological functions. AQ production in Pseudomonas aeruginosa is dependent on the activity of the gene pqsA, which converts anthranilate to anthraniloyl-CoA. Production of the resulting AQs is mediated by the activity of enzymes encoded by the pqs operon. |
|---|
|
Structure |
|
|---|
| Synonyms: | 3-hydroxy-3-octylquinoline-2,4(1H,3H)-dione |
|---|
|
Chemical Formula: |
C17H23NO3 |
|---|
| Average Molecular Weight: |
289.3694 |
|---|
| Monoisotopic Molecular
Weight: |
289.1678 |
|---|
| InChI Key: |
NRBMJXVDNAKWTI-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C17H23NO3/c1-2-3-4-5-6-9-12-17(21)15(19)13-10-7-8-11-14(13)18-16(17)20/h7-8,10-11,21H,2-6,9,12H2,1H3,(H,18,20) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | Not Available |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | O=C(C1=C(N2)C=CC=C1)C(O)(CCCCCCCC)C2=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as hydroxyquinolones. These are compounds containing a quinoline moiety bearing a hydroxyl group and a ketone. Quinoline or benzo[b]pyridine is a bicyclic compound that consists of benzene fused to a pyridine. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Quinolines and derivatives |
|---|
| Sub Class | Quinolones and derivatives |
|---|
|
Direct Parent |
Hydroxyquinolones |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hydroxyquinolone
- Dihydroquinolone
- Hydroxyquinoline
- Dihydroquinoline
- Hydroxypyridine
- Benzenoid
- Pyridine
- Heteroaromatic compound
- Vinylogous amide
- Azacycle
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
- quinolone (CHEBI:29472)
- a tautomer (CPD-12838)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | Not Available |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | Not Available |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Lepine F, Milot S, Deziel E, He J, Rahme LG. Electrospray/Mass Spectrometric Identification and Analysis of 4-Hydroxy-2-Alkylquinolines (HAQs) Produced by Pseudomonas aeruginosa . Appl Microbiol Biotechnol. 2010; 86(5):1323-36. Pubmed: 15144975
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
Not Available |
|---|