|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120600 |
|---|
|
Identification |
|---|
| Name: |
2-keto-L-gulonate |
|---|
| Description: | 2-Keto-L-gluconate is a derivative of gluconic acid, which occurs naturally in fruit, honey and wine and is used as a food additive, an acidity regulator. It is also used in cleaning products where it helps cleaning up mineral deposits. It is a strong chelating agent, especially in alkaline solution. It chelates the anions of calcium, iron, aluminium, copper, and other heavy metals. |
|---|
|
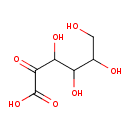
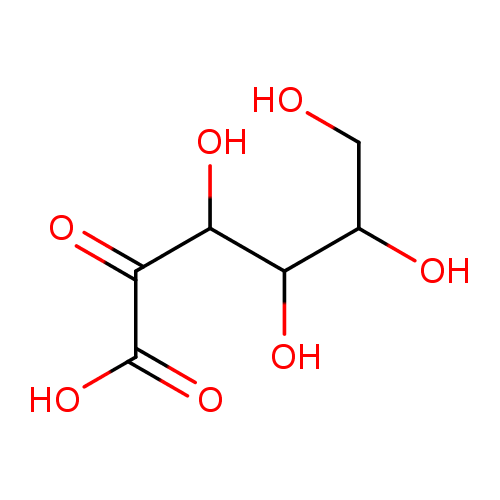
Structure |
|
|---|
| Synonyms: | - 2-dehydro-L-idonate
- 2-Dehydro-L-idonate
- 2-Keto-L-gulonate
- 2-Oxo-L-gulonate
- L-Sorbosonate
- L-xylo-hex-2-ulosonate
|
|---|
|
Chemical Formula: |
C6H9O7 |
|---|
| Average Molecular Weight: |
193.133 |
|---|
| Monoisotopic Molecular
Weight: |
194.04265 |
|---|
| InChI Key: |
VBUYCZFBVCCYFD-NUNKFHFFSA-M |
|---|
| InChI: | InChI=1S/C6H10O7/c7-1-2(8)3(9)4(10)5(11)6(12)13/h2-4,7-10H,1H2,(H,12,13)/p-1/t2-,3+,4-/m0/s1 |
|---|
| CAS
number: |
91548-32-2 |
|---|
| IUPAC Name: | L-sorbosonate |
|---|
|
Traditional IUPAC Name: |
2-keto-D-gluconic acid |
|---|
| SMILES: | C(C(C(C(C(CO)O)O)O)=O)([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as sugar acids and derivatives. These are compounds containing a saccharide unit which bears a carboxylic acid group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Sugar acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hexose monosaccharide
- Medium-chain keto acid
- Beta-hydroxy acid
- Sugar acid
- Acyloin
- Alpha-keto acid
- Beta-hydroxy ketone
- Hydroxy acid
- Keto acid
- Monosaccharide
- Alpha-hydroxy ketone
- Ketone
- Secondary alcohol
- Carboxylic acid
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Polyol
- Alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Primary alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|