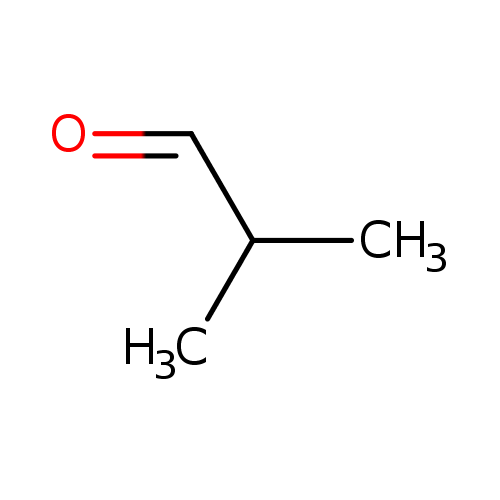|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120598 |
|---|
|
Identification |
|---|
| Name: |
isobutanal |
|---|
| Description: | A member of the class of propanals that is propanal substituted by a methyl group at position 2. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2-methylpropanal
- 2-methylpropionaldehyde
- α-methylpropionaldehyde
- isobutanal
- isobutylaldehyde
- isobutyric aldehyde
|
|---|
|
Chemical Formula: |
C4H8O |
|---|
| Average Molecular Weight: |
72.107 |
|---|
| Monoisotopic Molecular
Weight: |
72.05752 |
|---|
| InChI Key: |
AMIMRNSIRUDHCM-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C4H8O/c1-4(2)3-5/h3-4H,1-2H3 |
|---|
| CAS
number: |
78-84-2 |
|---|
| IUPAC Name: | 2-methylpropanal |
|---|
|
Traditional IUPAC Name: |
isobutyraldehyde |
|---|
| SMILES: | CC(C)[CH]=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as short-chain aldehydes. These are an aldehyde with a chain length containing between 2 and 5 carbon atoms. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organooxygen compounds |
|---|
|
Class |
Carbonyl compounds |
|---|
| Sub Class | Aldehydes |
|---|
|
Direct Parent |
Short-chain aldehydes |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hydrocarbon derivative
- Short-chain aldehyde
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
-65.9 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | -65.9 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 89 mg/mL at 25 °C | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Rodriguez GM, Atsumi S (2012)Isobutyraldehyde production from Escherichia coli by removing aldehyde reductase activity. Microbial cell factories 11, Pubmed: 22731523
- Lang K, Zierow J, Buehler K, Schmid A (2014)Metabolic engineering of Pseudomonas sp. strain VLB120 as platform biocatalyst for the production of isobutyric acid and other secondary metabolites. Microbial cell factories 13, Pubmed: 24397404
- Liu X, Bastian S, Snow CD, Brustad EM, Saleski TE, Xu JH, Meinhold P, Arnold FH (2012)Structure-guided engineering of Lactococcus lactis alcohol dehydrogenase LlAdhA for improved conversion of isobutyraldehyde to isobutanol. Journal of biotechnology 164, Pubmed: 22974724
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|