|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120591 |
|---|
|
Identification |
|---|
| Name: |
methionol |
|---|
| Description: | An alkyl sulfide that is propan-1-ol substituted by a methylsulfanyl group at position 3. It is a volatile compound found in wines and produced during fermentation. |
|---|
|
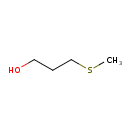
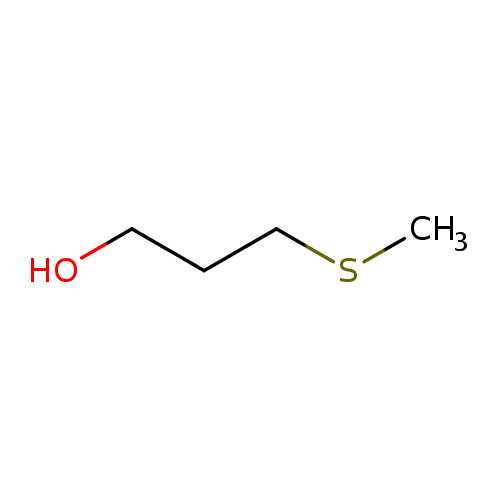
Structure |
|
|---|
| Synonyms: | - 3-(Methylthio)propyl alcohol
- 3-Hydroxypropyl methyl sulfide
- 3-Methylmercapto-1-propanol
- γ-Methylmercaptopropyl alcohol
- Methionol
|
|---|
|
Chemical Formula: |
C4H10OS |
|---|
| Average Molecular Weight: |
106.182 |
|---|
| Monoisotopic Molecular
Weight: |
106.045235 |
|---|
| InChI Key: |
CZUGFKJYCPYHHV-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C4H10OS/c1-6-4-2-3-5/h5H,2-4H2,1H3 |
|---|
| CAS
number: |
505-10-2 |
|---|
| IUPAC Name: | 3-(methylsulfanyl)propan-1-ol |
|---|
|
Traditional IUPAC Name: |
methionol |
|---|
| SMILES: | CSCCCO |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as dialkylthioethers. These are organosulfur compounds containing a thioether group that is substituted by two alkyl groups. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organosulfur compounds |
|---|
| Sub Class | Thioethers |
|---|
|
Direct Parent |
Dialkylthioethers |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Dialkylthioether
- Sulfenyl compound
- Organic oxygen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Seow YX, Ong PK, Liu SQ (2010)Production of flavour-active methionol from methionine metabolism by yeasts in coconut cream. International journal of food microbiology 143, Pubmed: 20805008
- Vallet A, Santarelli X, Lonvaud-Funel A, de Revel G, Cabanne C (2009)Purification of an alcohol dehydrogenase involved in the conversion of methional to methionol in Oenococcus oeni IOEB 8406. Applied microbiology and biotechnology 82, Pubmed: 18850096
- Silva Ferreira AC, Rodrigues P, Hogg T, Guedes De Pinho P (2003)Influence of some technological parameters on the formation of dimethyl sulfide, 2-mercaptoethanol, methionol, and dimethyl sulfone in port wines. Journal of agricultural and food chemistry 51, Pubmed: 12537449
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|