|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120586 |
|---|
|
Identification |
|---|
| Name: |
IDP |
|---|
| Description: | A nucleoside 5'-diphosphate(3−) arising from deprotonation of all three OH groups of the diphosphate function of of inosine 5'-diphosphate (IDP); major species at pH 7.3. |
|---|
|
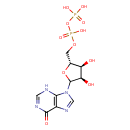
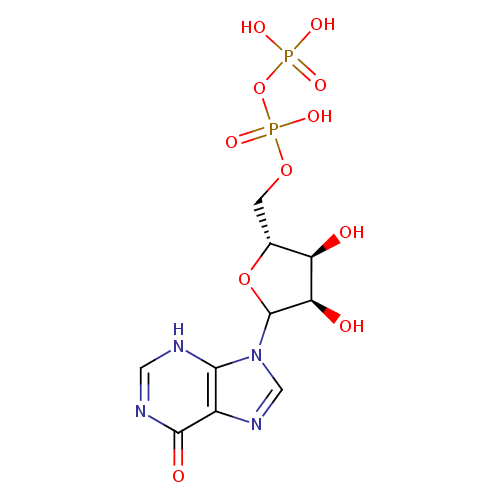
Structure |
|
|---|
| Synonyms: | - 5'-OO-[(phosphonatooxy)phosphinato]inosine
- IDP
- IDP trianion
- inosine 5'-diphosphate(3−)
|
|---|
|
Chemical Formula: |
C10H11N4O11P2 |
|---|
| Average Molecular Weight: |
425.165 |
|---|
| Monoisotopic Molecular
Weight: |
428.01343 |
|---|
| InChI Key: |
JPXZQMKKFWMMGK-KQYNXXCUSA-K |
|---|
| InChI: | InChI=1S/C10H14N4O11P2/c15-6-4(1-23-27(21,22)25-26(18,19)20)24-10(7(6)16)14-3-13-5-8(14)11-2-12-9(5)17/h2-4,6-7,10,15-16H,1H2,(H,21,22)(H,11,12,17)(H2,18,19,20)/p-3/t4-,6-,7-,10-/m1/s1 |
|---|
| CAS
number: |
86-04-4 |
|---|
| IUPAC Name: | inosine 5'-diphosphate |
|---|
|
Traditional IUPAC Name: |
{[(2R,3S,4R)-3,4-dihydroxy-5-(6-oxo-3H-purin-9-yl)oxolan-2-yl]methoxy(hydroxy)phosphoryl}oxyphosphonic acid |
|---|
| SMILES: | C(OP(=O)([O-])OP([O-])(=O)[O-])C1(OC(C(O)C(O)1)N3(C=NC2(C(=O)NC=NC=23))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as purine ribonucleoside diphosphates. These are purine ribobucleotides with diphosphate group linked to the ribose moiety. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
Purine ribonucleoside diphosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine ribonucleoside diphosphate
- Purine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-oxopurine
- Hypoxanthine
- Monosaccharide phosphate
- Organic pyrophosphate
- Imidazopyrimidine
- Purine
- Pyrimidone
- Monoalkyl phosphate
- Pyrimidine
- Alkyl phosphate
- Phosphoric acid ester
- Monosaccharide
- Organic phosphoric acid derivative
- N-substituted imidazole
- Vinylogous amide
- Azole
- Imidazole
- Oxolane
- Heteroaromatic compound
- Secondary alcohol
- 1,2-diol
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Alcohol
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Hlushko LV, Makovets'ka TI, Neiko IeM: [The dynamics of the glycocholic acid content of the bile in patients with chronic inactive hepatitis] Lik Sprava. 1995 May-Jun;(5-6):152-4. [8630789 ]
|
|---|
| Synthesis Reference: |
Takada, Masao; Kashiwa, Kenichi. Nucleoside 5'-pyrophosphates. Jpn. Tokkyo Koho (1971), 3 pp. CODEN: JAXXAD JP 46021587 19710618 Showa. CAN 75:130076 AN 1971:530076 CAPLUS |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|