|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120578 |
|---|
|
Identification |
|---|
| Name: |
ITP |
|---|
| Description: | A 2-hydroxy-3-methylpentanoate in which the stereocentres at positions 2 and 3 both have R-configuration. |
|---|
|
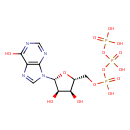
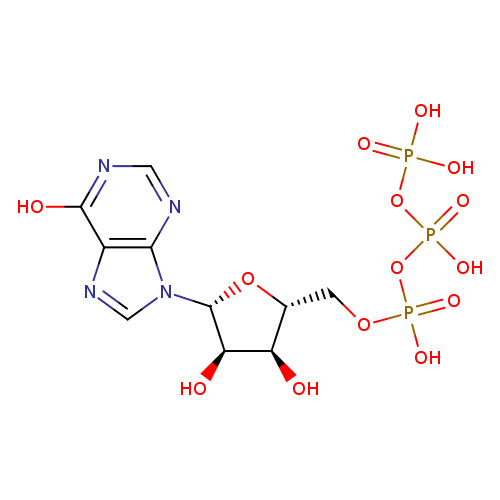
Structure |
|
|---|
| Synonyms: | - (2R,3R)-2-hydroxy-3-methylvalerate
- (2R,3R)-isoleucate
- (R,R)-isoleucate
- 2-hydroxy-3-methylpentanoate
- 2-hydroxy-3-methylvalerate
|
|---|
|
Chemical Formula: |
C10H11N4O14P3 |
|---|
| Average Molecular Weight: |
504.137 |
|---|
| Monoisotopic Molecular
Weight: |
507.97977 |
|---|
| InChI Key: |
HAEJPQIATWHALX-KQYNXXCUSA-J |
|---|
| InChI: | InChI=1S/C10H15N4O14P3/c15-6-4(1-25-30(21,22)28-31(23,24)27-29(18,19)20)26-10(7(6)16)14-3-13-5-8(14)11-2-12-9(5)17/h2-4,6-7,10,15-16H,1H2,(H,21,22)(H,23,24)(H,11,12,17)(H2,18,19,20)/p-4/t4-,6-,7-,10-/m1/s1 |
|---|
| CAS
number: |
132-06-9 |
|---|
| IUPAC Name: | ({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(6-hydroxy-9H-purin-9-yl)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)phosphonic acid |
|---|
|
Traditional IUPAC Name: |
({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(6-hydroxypurin-9-yl)oxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxyphosphonic acid |
|---|
| SMILES: | C(OP(=O)([O-])OP([O-])(=O)OP([O-])([O-])=O)C1(OC(C(O)C(O)1)N3(C=NC2(C(=O)NC=NC=23))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as purine ribonucleoside triphosphates. These are purine ribobucleotides with a triphosphate group linked to the ribose moiety. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
Purine ribonucleoside triphosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine ribonucleoside triphosphate
- Purine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-oxopurine
- Hypoxanthine
- Monosaccharide phosphate
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Pyrimidone
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Pyrimidine
- Oxolane
- Azole
- Vinylogous amide
- Heteroaromatic compound
- Imidazole
- Secondary alcohol
- 1,2-diol
- Azacycle
- Oxacycle
- Organoheterocyclic compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic nitrogen compound
- Alcohol
- Organonitrogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 903.5 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0a4r-0510090000-c39217a77098a2ef6d34 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-000i-3900000000-24a790aa14cc5baf5627 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-000i-3900000000-29bad6cebedb5d34080a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Lin S, McLennan AG, Ying K, Wang Z, Gu S, Jin H, Wu C, Liu W, Yuan Y, Tang R, Xie Y, Mao Y: Cloning, expression, and characterization of a human inosine triphosphate pyrophosphatase encoded by the itpa gene. J Biol Chem. 2001 Jun 1;276(22):18695-701. Epub 2001 Mar 13. [11278832 ]
- Sumi S, Marinaki AM, Arenas M, Fairbanks L, Shobowale-Bakre M, Rees DC, Thein SL, Ansari A, Sanderson J, De Abreu RA, Simmonds HA, Duley JA: Genetic basis of inosine triphosphate pyrophosphohydrolase deficiency. Hum Genet. 2002 Oct;111(4-5):360-7. Epub 2002 Aug 15. [12384777 ]
- Zachara B, Kopff M: Activity of inosine triphosphate pyrophosphohydrolase in fresh and stored human erythrocytes. Haematologia (Budap). 1981;14(3):277-83. [6120123 ]
- Vormittag W, Brannath W: As to the clastogenic-, sister-chromatid exchange inducing-and cytotoxic activity of inosine triphosphate in cultures of human peripheral lymphocytes. Mutat Res. 2001 May 9;476(1-2):71-81. [11336985 ]
- Dutta TK, Goel A, Ghotekar LH, Hamide A, Badhe BA, Basu D: Dapsone in treatment of chronic idiopathic thrombocytopenic purpura in adults. J Assoc Physicians India. 2001 Apr;49:421-5. [11762611 ]
- Soder C, Henderson JF, Zombor G, McCoy EE, Verhoef V, Morris AJ: Relationships between nucleoside triphosphate pyrophosphohydrolase activity and inosine triphosphate accumulation in human erythrocytes. Can J Biochem. 1976 Oct;54(10):843-7. [990987 ]
- Henderson JF, Zombor G, Fraser JH, McCoy EE, Verhoef V, Morris AJ: Factors affecting inosinate synthesis and inosine triphosphate accumulation in human erythrocytes. Can J Biochem. 1977 Apr;55(4):359-64. [15708 ]
- Klinker JF, Seifert R: Functionally nonequivalent interactions of guanosine 5'-triphosphate, inosine 5'-triphosphate, and xanthosine 5'-triphosphate with the retinal G-protein, transducin, and with Gi-proteins in HL-60 leukemia cell membranes. Biochem Pharmacol. 1997 Sep 1;54(5):551-62. [9337071 ]
- Zachara B, Lewandowski J: Isolation and identification of inosine triphosphate from human erythrocytes. Biochim Biophys Acta. 1974 Jun 27;353(2):253-9. [4842021 ]
- Mikami T, Yoshino Y, Ito A: Does a relationship exist between the urate pool in the body and lipid peroxidation during exercise? Free Radic Res. 2000 Jan;32(1):31-9. [10625215 ]
- Kopff M, Zachara B, Klem J, Zakrzewska I: [Accumulation of inosine triphosphate in human erythrocytes as a function of ITP-pyrophosphohydrolase activity] Acta Haematol Pol. 1983 Jul-Dec;14(3-4):165-71. [6147059 ]
- Fraser JH, Meyers H, Henderson JF, Brox LW, McCoy EE: Individual variation in inosine triphosphate accumulation in human erythrocytes. Clin Biochem. 1975 Dec;8(6):353-64. [1204209 ]
|
|---|
| Synthesis Reference: |
Nakayama, Kiyoshi; Tanaka, Haruo. Fermentative preparation of inosine di- and triphosphate. Jpn. Tokkyo Koho (1972), 2 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|