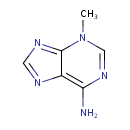
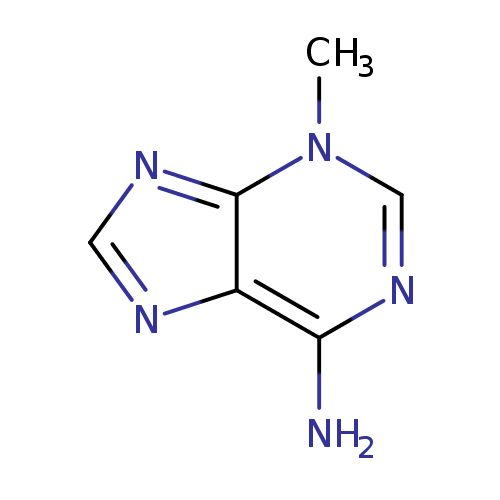
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (1 TMS) | splash10-0a4i-4980000000-d3e94e4150e2b870d9b2 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (1 TMS) | splash10-0a4i-3890000000-235f39119df7c967e9ba | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-0a4i-2980000000-251ceed13f156eff1fc4 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (1 TMS) | splash10-0ab9-9760000000-e3d1a7f774e6bfb718ae | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-0udi-0900000000-b32577a48ebafc94ae9a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-0udi-1900000000-485f684fffa023d0050e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-0udi-4900000000-6b3b3dd2f2f2d875bf14 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-0a59-9800000000-fe6c9700b9c081b8697a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-0udi-1900000000-b4a28f0f1bd551992615 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0udi-0900000000-8db506e73a05ea922d94 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) 30V, Positive | splash10-0udi-0900000000-f67b128eb2b6ac7139b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-53161ff2cee1a6ff34d7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0900000000-1ab536fe3bbb9bdfde76 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0giv-9600000000-a22dc2c9e956ce0b2b86 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-31965d8430668c1cef72 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006t-2900000000-1c6ebd899170870682a9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pc0-5900000000-23b69a04a094c620fe7f | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
| References: |
- Tavazzi B, Lazzarino G, Leone P, Amorini AM, Bellia F, Janson CG, Di Pietro V, Ceccarelli L, Donzelli S, Francis JS, Giardina B (2005)Simultaneous high performance liquid chromatographic separation of purines, pyrimidines, N-acetylated amino acids, and dicarboxylic acids for the chemical diagnosis of inborn errors of metabolism. Clinical biochemistry 38, Pubmed: 16139832
- Roux A, Xu Y, Heilier JF, Olivier MF, Ezan E, Tabet JC, Junot C (2012)Annotation of the human adult urinary metabolome and metabolite identification using ultra high performance liquid chromatography coupled to a linear quadrupole ion trap-Orbitrap mass spectrometer. Analytical chemistry 84, Pubmed: 22770225
- Li M, Yang X, Wang H, Xu E, Xi Z (2014)Inhibition of androgen induces autophagy in benign prostate epithelial cells. International journal of urology : official journal of the Japanese Urological Association 21, Pubmed: 23819759
- Huang YT, Cheng CC, Lin TC, Chiu TH, Lai PC (2014)Therapeutic potential of sepantronium bromide YM155 in gemcitabine-resistant human urothelial carcinoma cells. Oncology reports 31, Pubmed: 24297644
- Pickard RD, Spencer BH, McFarland AJ, Bernaitis N, Davey AK, Perkins AV, Chess-Williams R, McDermott CM, Forbes A, Christie D, Anoopkumar-Dukie S (2015)Paradoxical effects of the autophagy inhibitor 3-methyladenine on docetaxel-induced toxicity in PC-3 and LNCaP prostate cancer cells. Naunyn-Schmiedeberg's archives of pharmacology 388, Pubmed: 25708950
- Tran AT, Ramalinga M, Kedir H, Clarke R, Kumar D (2015)Autophagy inhibitor 3-methyladenine potentiates apoptosis induced by dietary tocotrienols in breast cancer cells. European journal of nutrition 54, Pubmed: 24830781
|
|---|