|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120567 |
|---|
|
Identification |
|---|
| Name: |
3,4-dihydroxyphenylglycol |
|---|
| Description: | A tetrol composed of ethyleneglycol having a 3,4-dihydroxyphenyl group at the 1-position. |
|---|
|
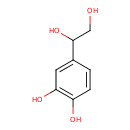
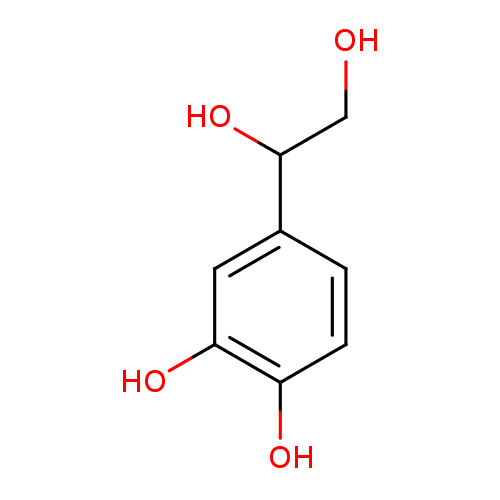
Structure |
|
|---|
| Synonyms: | - (3,4-dihydroxyphenyl)ethylene glycol
- 1-(3,4-dihydroxyphenyl)-1,2-ethanediol
- 2-hydroxy-2-(3,4-dihydroxy)phenylethanol
- 3,4-dihydroxyphenethyl glycol
- 3,4-dihydroxyphenylethyl glycol
- 3,4-dihydroxyphenylethyleneglycol
- 3,4-Dihydroxyphenylglycol
- β,3,4-trihydroxy phenethyl alcohol
- DHPG
- Dihydroxyphenylethylene glycol
- DOPEG
|
|---|
|
Chemical Formula: |
C8H10O4 |
|---|
| Average Molecular Weight: |
170.165 |
|---|
| Monoisotopic Molecular
Weight: |
170.0579 |
|---|
| InChI Key: |
MTVWFVDWRVYDOR-QMMMGPOBSA-N |
|---|
| InChI: | InChI=1S/C8H10O4/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8-12H,4H2/t8-/m0/s1 |
|---|
| CAS
number: |
28822-73-3 |
|---|
| IUPAC Name: | 4-(1,2-dihydroxyethyl)benzene-1,2-diol |
|---|
|
Traditional IUPAC Name: |
3,4-dihydroxyphenylglycol |
|---|
| SMILES: | C(O)C(O)C1(C=CC(O)=C(O)C=1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as catechols. These are compounds containing a 1,2-benzenediol moiety. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Benzenoids |
|---|
| Sub Class | Phenols |
|---|
|
Direct Parent |
Catechols |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Catechol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Secondary alcohol
- 1,2-diol
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic alcohol
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
130 - 132 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 130 - 132 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | -1.01 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (4 TMS) | splash10-0a59-0965000000-0c949d05cfff1600a5db | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-00fr-2900000000-fbceba2f96c4ee1154ae | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-000i-9300000000-dd28ad7f56904a5c7c7e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0bvi-9200000000-b9f4f1a5509344427d53 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-52) , Positive | splash10-00y3-8900000000-0cb05419b964f5d6016b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-785c9bdff21d7558b9b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fk9-1900000000-953cf944dd232b8d7832 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zg0-7900000000-cb8414fe82a1c814f764 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-a3cd8f79f0fba00900c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0aor-1900000000-ecff175233ea65fe5f8f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-7900000000-f8265f1946f33cedd546 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Medina E, de Castro A, Romero C, Brenes M (2006)Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: correlation with antimicrobial activity. Journal of agricultural and food chemistry 54, Pubmed: 16819902
- Roux A, Xu Y, Heilier JF, Olivier MF, Ezan E, Tabet JC, Junot C (2012)Annotation of the human adult urinary metabolome and metabolite identification using ultra high performance liquid chromatography coupled to a linear quadrupole ion trap-Orbitrap mass spectrometer. Analytical chemistry 84, Pubmed: 22770225
- DellaGreca M, Monaco P, Pinto G, Pollio A, Previtera L, Temussi F (2001)Phytotoxicity of low-molecular-weight phenols from olive mill waste waters. Bulletin of environmental contamination and toxicology 67, Pubmed: 11479664
- Holmes LJ, Storlien LH, Smythe GA (1989)Hypothalamic monoamines associated with the cephalic phase insulin response. The American journal of physiology 256, Pubmed: 2645784
- Howes LG, Reid JL (1985)Decreased vascular responsiveness to noradrenaline following regular ethanol consumption. British journal of clinical pharmacology 20, Pubmed: 4091997
- Soares-da-Silva P, Caramona MM (1988)Effects of methylene blue on the uptake, release and metabolism of noradrenaline in mesenteric arterial vessels. The Journal of pharmacy and pharmacology 40, Pubmed: 2907005
- Head RJ, Cassis LA, Barone S, Stitzel RE, de la Lande IS (1984)Neuronal deamination of endogenous and exogenous noradrenaline in the mesenteric artery of the spontaneously hypertensive rat. The Journal of pharmacy and pharmacology 36, Pubmed: 6146669
- Minson JB, de la Lande IS (1984)Metabolism of exogenous noradrenaline in slices of hypothalamus and caudate nucleus of the brush-tail possum, Trichosurus vulpecula. The Australian journal of experimental biology and medical science 62 ( Pt 3), Pubmed: 6497782
- Lôo H, Scatton B, Dennis T, Benkelfat C, Gay C, Poirier-Littré MF, Garreau M, Vanelle JM, Olié JP, Deniker P (1983)[Study of noradrenaline metabolism in depressed patients by the determination of plasma dihydroxyphenylethylene glycol]. L'Encephale 9, Pubmed: 6671452
- Howes LG, Hodsman GP, Maccarrone C, Kohzuki M, Johnston CI (1989)Cardiac 3,4-dihydroxyphenylethylene glycol (DHPG) and catecholamine levels in a rat model of left ventricular failure. Journal of cardiovascular pharmacology 13, Pubmed: 2468969
- Isidori M, Lavorgna M, Nardelli A, Parrella A (2005)Model study on the effect of 15 phenolic olive mill wastewater constituents on seed germination and Vibrio fischeri metabolism. Journal of agricultural and food chemistry 53, Pubmed: 16218695
- Elsworth JD, Roth RH, Redmond DE (1983)Relative importance of 3-methoxy-4-hydroxyphenylglycol and 3,4-dihydroxyphenylglycol as norepinephrine metabolites in rat, monkey, and humans. Journal of neurochemistry 41, Pubmed: 6875564
- Jackman G, Snell J, Skews H, Bobik A (1982)Effects of noradrenergic neuronal activity on 3,4-dihydroxyphenylethylene glycol (DHPG) levels. Quantitation by high performance liquid chromatography. Life sciences 31, Pubmed: 7176821
- Loo H, Scatton B, Poirier MF, Benkelfat C, Dennis T, Vanelle JM, Garreau M, Sechter D (1985)[Study of the metabolism of cerebral noradrenaline in depressed patients by the assay of plasma dihydroxyphenylethylene glycol]. Presse medicale (Paris, France : 1983) 14, Pubmed: 3161030
|
|---|
| Synthesis Reference: |
Hunter L W; Rorie D K; Yaksh T L; Tyce G M Concurrent separation of catecholamines, dihydroxyphenylglycol, vasoactive intestinal peptide, and neuropeptide Y in superfusate and tissue extract. Analytical biochemistry (1988), 173(2), 340-52. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|