|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120566 |
|---|
|
Identification |
|---|
| Name: |
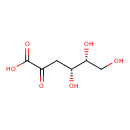
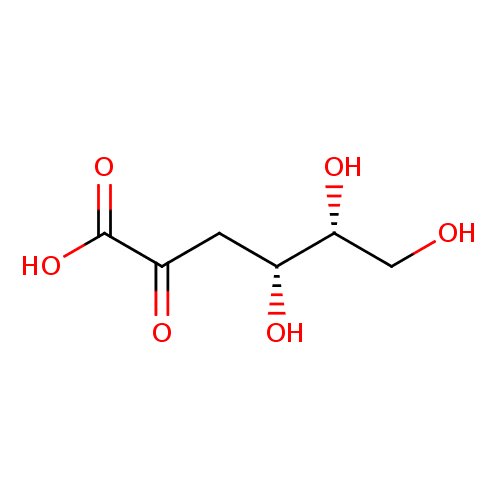
2-dehydro-3-deoxy-D-gluconate |
|---|
| Description: | The conjugate base of 2-dehydro-3-deoxy-D-gluconic acid; major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2-dehydro-3-deoxy-D-gluconate
- 2-dehydro-3-deoxy-D-gluconate anion
- 2-dehydro-3-deoxy-D-gluconate(1−)
|
|---|
|
Chemical Formula: |
C6H9O6 |
|---|
| Average Molecular Weight: |
177.133 |
|---|
| Monoisotopic Molecular
Weight: |
178.04774 |
|---|
| InChI Key: |
WPAMZTWLKIDIOP-WVZVXSGGSA-M |
|---|
| InChI: | InChI=1S/C6H10O6/c7-2-5(10)3(8)1-4(9)6(11)12/h3,5,7-8,10H,1-2H2,(H,11,12)/p-1/t3-,5+/m0/s1 |
|---|
| CAS
number: |
17510-99-5 |
|---|
| IUPAC Name: | 3-deoxy-D-erythro-hex-2-ulosonate |
|---|
|
Traditional IUPAC Name: |
(4R,5R)-4,5,6-trihydroxy-2-oxohexanoic acid |
|---|
| SMILES: | C(=O)([O-])C(=O)CC(O)C(O)CO |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as medium-chain keto acids and derivatives. These are keto acids with a 6 to 12 carbon atoms long side chain. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Keto acids and derivatives |
|---|
|
Direct Parent |
Medium-chain keto acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Medium-chain keto acid
- Alpha-keto acid
- Beta-hydroxy ketone
- Alpha-hydroxy ketone
- Secondary alcohol
- Ketone
- Polyol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Primary alcohol
- Organooxygen compound
- Organic oxygen compound
- Carbonyl group
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kim S, Lee SB (2006)Characterization of Sulfolobus solfataricus 2-keto-3-deoxy-D-gluconate kinase in the modified Entner-Doudoroff pathway. Bioscience, biotechnology, and biochemistry 70, Pubmed: 16794308
|
|---|
| Synthesis Reference: |
Plantier-Royon, Richard; Anker, Daniel; Robert-Baudouy, , Janine. New synthesis of 3-deoxy-D-erythro-2-hexulosonic acid (KDG) from D-glucose. Journal of Carbohydrate Chemistry (1991), 10(2), 239-49. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|