|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120555 |
|---|
|
Identification |
|---|
| Name: |
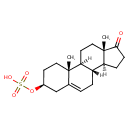
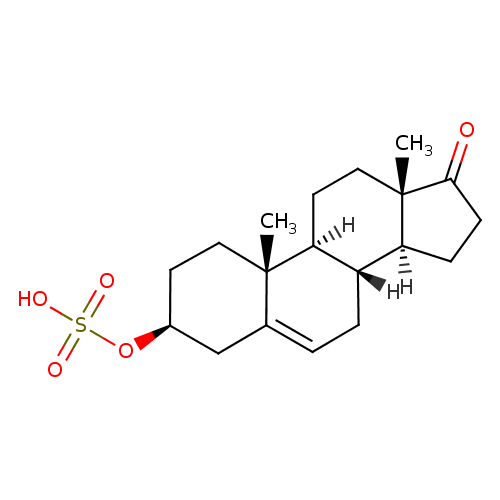
3-β-hydroxyandrost-5-en-17-one 3-sulfate |
|---|
| Description: | A steroid sulfate that is the 3-sulfooxy derivative of dehydroepiandrosterone. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (3-beta)-3-(Sulfooxy)androst-5-en-17-one
- 17-Ketoandrost-5-en-3beta-yl sulfate
- 17-oxoandrost-5-en-3β-yl hydrogen sulphate
- 3-O-Sulfodehydroepiandrosterone
- 3beta-Hydroxyandrost-5-en-17-one 3-sulfate
- 3beta-hydroxyandrost-5-en-17-one 3-sulfate
- 3beta-Hydroxyandrost-5-en-17-one 3-sulfate
- Androst-5-en-17-on-3beta-yl sulfuric acid
- Dehydroepiandrosterone 3-sulfate
- Dehydroepiandrosterone monosulfate
- Dehydroepiandrosterone sulfate
- Dehydroepiandrosterone sulphate
- Dehydroisoandrosterone sulfate
- Dehydroisoandrosterone-3-sulfate
- DHEA sulfate
- DHEA sulfate
- DHEA-S
- DHEAS
- Prasterone sulfate
|
|---|
|
Chemical Formula: |
C19H29O5S |
|---|
| Average Molecular Weight: |
369.495 |
|---|
| Monoisotopic Molecular
Weight: |
370.1814 |
|---|
| InChI Key: |
ZMITXKRGXGRMKS-QRIARFFBSA-M |
|---|
| InChI: | InChI=1S/C19H30O5S/c1-18-9-7-13(24-25(21,22)23)11-12(18)3-4-14-15-5-6-17(20)19(15,2)10-8-16(14)18/h12-16H,3-11H2,1-2H3,(H,21,22,23)/p-1/t12?,13-,14-,15-,16-,18-,19-/m0/s1 |
|---|
| CAS
number: |
651-48-9 |
|---|
| IUPAC Name: | 17-oxoandrost-5-en-3β-yl hydrogen sulfate |
|---|
|
Traditional IUPAC Name: |
dehydroepiandrosterone sulfate |
|---|
| SMILES: | CC24(CCC(CC(CC[CH]1([CH]3(CCC(=O)C(C)(CC[CH]12)3)))4)OS(=O)(=O)[O-]) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as sulfated steroids. These are sterol lipids containing a sulfate group attached to the steroid skeleton. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Steroids and steroid derivatives |
|---|
| Sub Class | Sulfated steroids |
|---|
|
Direct Parent |
Sulfated steroids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Sulfated steroid skeleton
- Androstane-skeleton
- 17-oxosteroid
- Oxosteroid
- Delta-5-steroid
- Sulfuric acid ester
- Alkyl sulfate
- Sulfuric acid monoester
- Sulfate-ester
- Organic sulfuric acid or derivatives
- Ketone
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxygen compound
- Carbonyl group
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework |
Aliphatic homopolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Androgen and Estrogen Metabolism pae00150
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Barrou Z, Charru P, Lidy C (1997)Dehydroepiandrosterone (DHEA) and aging. Archives of gerontology and geriatrics 24, Pubmed: 15374110
- Sato H, Macchiarulo A, Thomas C, Gioiello A, Une M, Hofmann AF, Saladin R, Schoonjans K, Pellicciari R, Auwerx J (2008)Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. Journal of medicinal chemistry 51, Pubmed: 18307294
- Roux A, Xu Y, Heilier JF, Olivier MF, Ezan E, Tabet JC, Junot C (2012)Annotation of the human adult urinary metabolome and metabolite identification using ultra high performance liquid chromatography coupled to a linear quadrupole ion trap-Orbitrap mass spectrometer. Analytical chemistry 84, Pubmed: 22770225
- Hemminki A, Niemi S, Hautoniemi L, Söderlund H, Takkinen K (1998)Fine tuning of an anti-testosterone antibody binding site by stepwise optimisation of the CDRs. Immunotechnology : an international journal of immunological engineering 4, Pubmed: 9661815
- Leonardi R, Zhang YM, Yun MK, Zhou R, Zeng FY, Lin W, Cui J, Chen T, Rock CO, White SW, Jackowski S (2010)Modulation of pantothenate kinase 3 activity by small molecules that interact with the substrate/allosteric regulatory domain. Chemistry & biology 17, Pubmed: 20797618
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|