|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120505 |
|---|
|
Identification |
|---|
| Name: |
palmitoyl-CoA |
|---|
| Description: | A saturated fatty acyl-CoA(4−) that is palmitoyl-CoA in which the phosphate and diphosphate groups have been deprotonated to give the corresponding tetra-anion. |
|---|
|
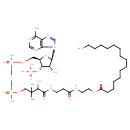
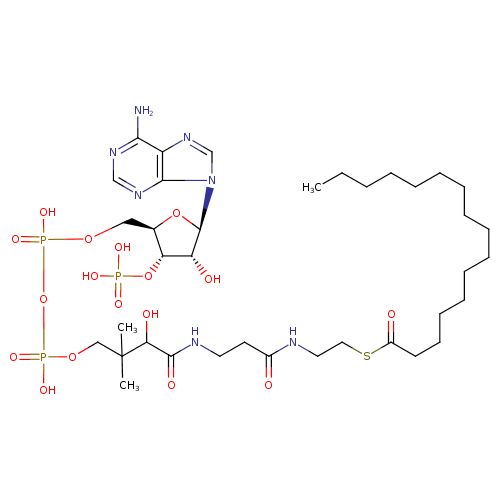
Structure |
|
|---|
| Synonyms: | - Palmitoyl-CoA
- (n-C16:0CoA)
- Hexadecanoyl CoA
- Palmityl-CoA
- Palmitoyl coenzyme-A
- Palmitoyl CoA
|
|---|
|
Chemical Formula: |
C37H62N7O17P3S |
|---|
| Average Molecular Weight: |
1001.915 |
|---|
| Monoisotopic Molecular
Weight: |
1005.34485 |
|---|
| InChI Key: |
MNBKLUUYKPBKDU-BBECNAHFSA-J |
|---|
| InChI: | InChI=1S/C37H66N7O17P3S/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-28(46)65-21-20-39-27(45)18-19-40-35(49)32(48)37(2,3)23-58-64(55,56)61-63(53,54)57-22-26-31(60-62(50,51)52)30(47)36(59-26)44-25-43-29-33(38)41-24-42-34(29)44/h24-26,30-32,36,47-48H,4-23H2,1-3H3,(H,39,45)(H,40,49)(H,53,54)(H,55,56)(H2,38,41,42)(H2,50,51,52)/p-4/t26-,30-,31-,32+,36-/m1/s1 |
|---|
| CAS
number: |
1763-10-6 |
|---|
| IUPAC Name: | 3'- phosphonatoadenosine 5'- phosphonatoadenosine 5'- (3- (3- {(3R)- {(3R)- 4- 4- [(3- [(3- {[2- {[2- (hexadecanoylsulfanyl)ethyl]amino}- (hexadecanoylsulfanyl)ethyl]amino}- 3- 3- oxopropyl)amino]- oxopropyl)amino]- 3- 3- hydroxy- hydroxy- 2,2- 2,2- dimethyl- dimethyl- 4- 4- oxobutyl} diphosphate) oxobutyl} diphosphate) |
|---|
|
Traditional IUPAC Name: |
[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-2-{[({3-[(2-{[2-(hexadecanoylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]-3-hydroxy-2,2-dimethylpropoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]methyl}-4-hydroxyoxolan-3-yl]oxyphosphonic acid |
|---|
| SMILES: | CCCCCCCCCCCCCCCC(SCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(=O)(OP(=O)(OCC1(C(OP([O-])(=O)[O-])C(O)C(O1)N3(C2(=C(C(N)=NC=N2)N=C3))))[O-])[O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as long-chain fatty acyl coas. These are acyl CoAs where the group acylated to the coenzyme A moiety is a long aliphatic chain of 13 to 21 carbon atoms. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Lipids and lipid-like molecules |
|---|
| Sub Class | Fatty Acyls |
|---|
|
Direct Parent |
Long-chain fatty acyl CoAs |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Pentose phosphate
- Pentose-5-phosphate
- Ribonucleoside 3'-phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Organic pyrophosphate
- 6-aminopurine
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Aminopyrimidine
- Fatty amide
- Imidolactam
- Monosaccharide
- N-acyl-amine
- N-substituted imidazole
- Organic phosphoric acid derivative
- Alkyl phosphate
- Phosphoric acid ester
- Primary aromatic amine
- Pyrimidine
- Oxolane
- Azole
- Imidazole
- Heteroaromatic compound
- Amino acid or derivatives
- Thiocarboxylic acid ester
- Carboxamide group
- Carbothioic s-ester
- Secondary carboxylic acid amide
- Secondary alcohol
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Carboxylic acid derivative
- Hydrocarbon derivative
- Alcohol
- Organic nitrogen compound
- Amine
- Organonitrogen compound
- Carbonyl group
- Organooxygen compound
- Organosulfur compound
- Organic oxygen compound
- Organopnictogen compound
- Primary amine
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA: Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes. 2005 Nov;54(11):3148-53. [16249438 ]
- Gohil K, Jones DA, Edwards RH: Fatty acid oxidation in mitochondria from needle biopsy samples of human skeletal muscle. Clin Sci (Lond). 1984 Feb;66(2):173-8. [6319070 ]
- Berge RK, Hagen LE, Farstad M: Isolation of palmitoyl-CoA hydrolases from human blood platelets. Biochem J. 1981 Dec 1;199(3):639-47. [6122441 ]
- Casteels M, Schepers L, Parmentier G, Eyssen HJ, Mannaerts GP: Activation and peroxisomal beta-oxidation of fatty acids and bile acid intermediates in liver from Bombina orientalis and from the rat. Comp Biochem Physiol B. 1989;92(1):129-32. [2706931 ]
- Carroll JE, McGuire BS, Hall CL: Fatty acyl-CoA dehydrogenase enzymes in human skeletal muscle. Clin Chim Acta. 1986 Dec 30;161(3):327-33. [3802539 ]
- Holloway GP, Bezaire V, Heigenhauser GJ, Tandon NN, Glatz JF, Luiken JJ, Bonen A, Spriet LL: Mitochondrial long chain fatty acid oxidation, fatty acid translocase/CD36 content and carnitine palmitoyltransferase I activity in human skeletal muscle during aerobic exercise. J Physiol. 2006 Feb 15;571(Pt 1):201-10. Epub 2005 Dec 15. [16357012 ]
- Bakken AM, Farstad M: Identical subcellular distribution of palmitoyl-CoA and arachidonoyl-CoA synthetase activities in human blood platelets. Biochem J. 1989 Jul 1;261(1):71-6. [2528345 ]
- Fukao T, Watanabe H, Orii K, Takahashi Y, Hirano A, Kondo T, Yamaguchi S, Aoyama T, Kondo N: Myopathic form of very-long chain acyl-coa dehydrogenase deficiency: evidence for temperature-sensitive mild mutations in both mutant alleles in a Japanese girl. Pediatr Res. 2001 Feb;49(2):227-31. [11158518 ]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP: Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004 Feb;55(2):257-67. [14755730 ]
- Tonsgard JH, Stephens JK, Rhead WJ, Penn D, Horwitz AL, Kirschner BS, Whitington PF, Berger S, Tripp ME: Defect in fatty acid oxidation: laboratory and pathologic findings in a patient. Pediatr Neurol. 1991 Mar-Apr;7(2):125-30. [2059253 ]
- Wanders RJ, van Roermund CW, de Vries CT, van den Bosch H, Schrakamp G, Tager JM, Schram AW, Schutgens RB: Peroxisomal beta-oxidation of palmitoyl-CoA in human liver homogenates and its deficiency in the cerebro-hepato-renal (Zellweger) syndrome. Clin Chim Acta. 1986 Aug 30;159(1):1-10. [2944672 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|