|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120503 |
|---|
|
Identification |
|---|
| Name: |
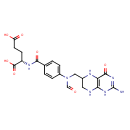
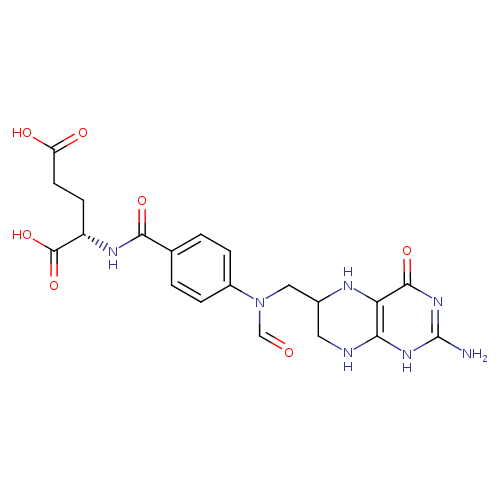
10-formyl-tetrahydrofolate |
|---|
| Description: | Dianion of 10-formyltetrahydrofolic acid arising from deprotonation of both carboxylic acid functions. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 10-formyltetrahydrofolate
- 10-formyltetrahydrofolate dianion
|
|---|
|
Chemical Formula: |
C20H21N7O7 |
|---|
| Average Molecular Weight: |
471.429 |
|---|
| Monoisotopic Molecular
Weight: |
473.1659 |
|---|
| InChI Key: |
AUFGTPPARQZWDO-YUZLPWPTSA-L |
|---|
| InChI: | InChI=1S/C20H23N7O7/c21-20-25-16-15(18(32)26-20)23-11(7-22-16)8-27(9-28)12-3-1-10(2-4-12)17(31)24-13(19(33)34)5-6-14(29)30/h1-4,9,11,13,23H,5-8H2,(H,24,31)(H,29,30)(H,33,34)(H4,21,22,25,26,32)/p-2/t11?,13-/m0/s1 |
|---|
| CAS
number: |
2800-34-2 |
|---|
| IUPAC Name: | N- [4- [4- (N- (N- {[(6S)- {[(6S)- 2- 2- amino- amino- 4- 4- oxo- oxo- 3,4,5,6,7,8- 3,4,5,6,7,8- hexahydropteridin- hexahydropteridin- 6- 6- yl]methyl}formamido)benzoyl]- yl]methyl}formamido)benzoyl]- L- L- glutamate glutamate |
|---|
|
Traditional IUPAC Name: |
(2S)-2-[(4-{N-[(2-amino-4-oxo-5,6,7,8-tetrahydro-1H-pteridin-6-yl)methyl]formamido}phenyl)formamido]pentanedioic acid |
|---|
| SMILES: | C2(C(CN(C=O)C1(C=CC(C(=O)NC(C(=O)[O-])CCC([O-])=O)=CC=1))NC3(C(=O)NC(N)=NC(N2)=3)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as tetrahydrofolic acids. These are heterocyclic compounds based on the 5,6,7,8-tetrahydropteroic acid skeleton conjugated with at least one L-glutamic acid unit. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Pteridines and derivatives |
|---|
|
Direct Parent |
Tetrahydrofolic acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Tetrahydrofolic acid
- Glutamic acid or derivatives
- Acylaminobenzoic acid or derivatives
- Hippuric acid or derivatives
- Hippuric acid
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid or derivatives
- Benzamide
- Benzoic acid or derivatives
- Anilide
- Benzoyl
- Aminopyrimidine
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Monocyclic benzene moiety
- Dicarboxylic acid or derivatives
- Primary aromatic amine
- Pyrimidine
- Benzenoid
- Tertiary carboxylic acid amide
- Vinylogous amide
- Heteroaromatic compound
- Amino acid or derivatives
- Secondary carboxylic acid amide
- Amino acid
- Carboxamide group
- Secondary amine
- Azacycle
- Carboxylic acid derivative
- Carboxylic acid
- Primary amine
- Carbonyl group
- Organonitrogen compound
- Organic oxide
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Amine
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Xu L, Li C, Olson AJ, Wilson IA: Crystal structure of avian aminoimidazole-4-carboxamide ribonucleotide transformylase in complex with a novel non-folate inhibitor identified by virtual ligand screening. J Biol Chem. 2004 Nov 26;279(48):50555-65. Epub 2004 Sep 7. [15355974 ]
- Baggott JE, Tamura T: Bioactivity of orally administered unnatural isomers, [6R]-5-formyltetrahydrofolate and [6S]-5,10-methenyltetrahydrofolate, in humans. Biochim Biophys Acta. 1999 Oct 18;1472(1-2):323-32. [10572954 ]
- Johlin FC, Swain E, Smith C, Tephly TR: Studies on the mechanism of methanol poisoning: purification and comparison of rat and human liver 10-formyltetrahydrofolate dehydrogenase. Mol Pharmacol. 1989 Jun;35(6):745-50. [2733692 ]
- Kirksey TJ, Appling DR: Site-directed mutagenesis of a highly conserved aspartate in the putative 10-formyl-tetrahydrofolate binding site of yeast C1-tetrahydrofolate synthase. Arch Biochem Biophys. 1996 Sep 1;333(1):251-9. [8806778 ]
- Baggott JE, Robinson CB, Johnston KE: Bioactivity of [6R]-5-formyltetrahydrofolate, an unusual isomer, in humans and Enterococcus hirae, and cytochrome c oxidation of 10-formytetrahydrofolate to 10-formyldihydrofolate. Biochem J. 2001 Feb 15;354(Pt 1):115-22. [11171086 ]
- Boger DL, Labroli MA, Marsilje TH, Jin Q, Hedrick MP, Baker SJ, Shim JH, Benkovic SJ: Conformationally restricted analogues designed for selective inhibition of GAR Tfase versus thymidylate synthase or dihydrofolate reductase. Bioorg Med Chem. 2000 May;8(5):1075-86. [10882019 ]
|
|---|
| Synthesis Reference: |
Denis V; Daignan-Fornier B Synthesis of glutamine, glycine and 10-formyl tetrahydrofolate is coregulated with purine biosynthesis in Saccharomyces cerevisiae. Molecular & general genetics : MGG (1998), 259(3), 246-55. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|