|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120487 |
|---|
|
Identification |
|---|
| Name: |
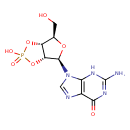
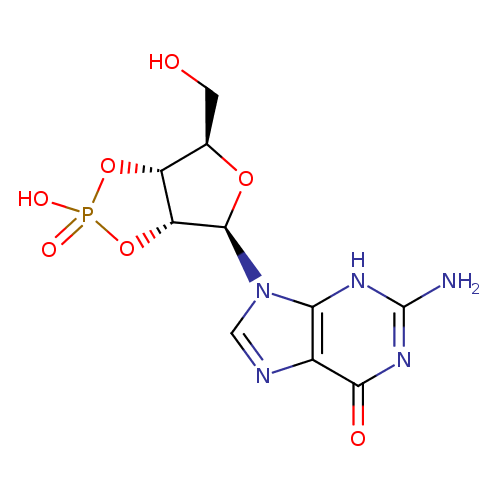
guanosine 2',3'-cyclic monophosphate |
|---|
| Description: | A 2',3'-cyclic nucleotide(1−) which is obtained from 2',3'-cyclic GMP by removal of a proton from the cyclic phosphate group. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2',3'-cyclic GMP
- cGMP(1−)
- cyclic guanosine 2',3'-monophosphate(1−)
- guanosine 2',3'-cyclic monophosphate(1−)
- guanosine 2',3'-cyclic phosphate(1−)
- guanosine cyclic-2',3'-monophosphate(1−)
- O2',O3'-
 hydroxyphosphoryl- hydroxyphosphoryl- guanosine(1−) guanosine(1−)
|
|---|
|
Chemical Formula: |
C10H11N5O7P |
|---|
| Average Molecular Weight: |
344.2 |
|---|
| Monoisotopic Molecular
Weight: |
345.04742 |
|---|
| InChI Key: |
UASRYODFRYWBRC-UUOKFMHZSA-M |
|---|
| InChI: | InChI=1S/C10H12N5O7P/c11-10-13-7-4(8(17)14-10)12-2-15(7)9-6-5(3(1-16)20-9)21-23(18,19)22-6/h2-3,5-6,9,16H,1H2,(H,18,19)(H3,11,13,14,17)/p-1/t3-,5-,6-,9-/m1/s1 |
|---|
| CAS
number: |
15718-49-7 |
|---|
| IUPAC Name: | (3aR,4R,6R,6aR)- 4- 4- (2- (2- amino- amino- 6- 6- oxo- oxo- 1,6- 1,6- dihydro- dihydro- 9H- 9H- purin- purin- 9- 9- yl)- yl)- 6- 6- (hydroxymethyl)tetrahydrofuro[3,4- (hydroxymethyl)tetrahydrofuro[3,4- d][1,3,2]dioxaphosphol- d][1,3,2]dioxaphosphol- 2- 2- olate 2- olate 2- oxide oxide |
|---|
|
Traditional IUPAC Name: |
9-[(3aR,4R,6R,6aR)-2-hydroxy-6-(hydroxymethyl)-2-oxo-tetrahydro-2???furo[3,4-d][1,3,2]dioxaphosphol-4-yl]-2-amino-3H-purin-6-one |
|---|
| SMILES: | C2(=NC1(C(=O)NC(N)=NC=1N2C4(OC(CO)C3(OP(=O)([O-])OC34)))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as 2',3'-cyclic purine nucleotides. These are purine nucleotides in which the oxygen atoms linked to the C2 and C3 carbon atoms of the ribose moiety are both bonded the same phosphorus atom of the phosphate group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
2',3'-cyclic purine nucleotides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 2',3'-cyclic purine ribonucleotide
- Ribonucleoside 3'-phosphate
- Imidazopyrimidine
- Purine
- Hydroxypyrimidine
- Monosaccharide
- N-substituted imidazole
- Pyrimidine
- Organic phosphoric acid derivative
- Azole
- 1,3_dioxaphospholane
- Heteroaromatic compound
- Imidazole
- Tetrahydrofuran
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Alcohol
- Organic oxygen compound
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organic oxide
- Organonitrogen compound
- Organooxygen compound
- Primary alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Genschik P, Billy E, Swianiewicz M, Filipowicz W: The human RNA 3'-terminal phosphate cyclase is a member of a new family of proteins conserved in Eucarya, Bacteria and Archaea. EMBO J. 1997 May 15;16(10):2955-67. [9184239 ]
- Filipowicz W, Billy E, Drabikowski K, Genschik P: Cyclases of the 3'-terminal phosphate in RNA: a new family of RNA processing enzymes conserved in eucarya, bacteria and archaea. Acta Biochim Pol. 1998;45(4):895-906. [10397337 ]
- Hamada K, Yokoro K: Blocking of DNA synthesis in vitro by a guanosine 2',3'-cyclic phosphate: a possible mechanism of chromosome aberrations induced by U5 snRNA. Mutat Res. 1995 Jan;326(1):71-82. [7528887 ]
- Diamantstein T, Ulmer A: Effect of cyclic nucleotides on DNA synthesis in mouse lymphoid cells. Immunol Commun. 1975;4(1):51-62. [163786 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|