|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120468 |
|---|
|
Identification |
|---|
| Name: |
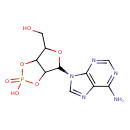
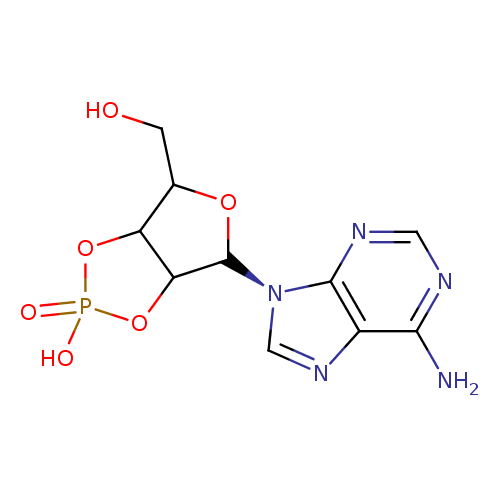
adenosine 2',3'-cyclic monophosphate |
|---|
| Description: | An organophosphate oxoanion which is obtained from 2',3'-cyclic AMP by removal of a proton from the cyclic phosphate group. |
|---|
|
Structure |
|
|---|
| Synonyms: | - Cyclic 2',3'-AMP
- 2',3'-Cyclic AMP
- adenosine 2',3'-(hydrogen phosphate)
- (2R,3aR,4R,6R,6aR)-4-(6-amino-9H-purin-9-yl)-6-(hydroxymethyl)tetrahydrofuro[3,4-d][1,3,2]dioxaphosphol-2-ol 2-oxide
- (3aR,4R,6R,6aR)-4-(6-amino-9H-purin-9-yl)-6-(hydroxymethyl)tetrahydrofuro[3,4-d][1,3,2]dioxaphosphol-2-ol
- 2-oxideadenosine cyclic 2',3'-(hydrogen phosphate)
- cyclic 2',3'-adenosine monophosphate
|
|---|
|
Chemical Formula: |
C10H11N5O6P |
|---|
| Average Molecular Weight: |
328.201 |
|---|
| Monoisotopic Molecular
Weight: |
329.05252 |
|---|
| InChI Key: |
KMYWVDDIPVNLME-KQYNXXCUSA-M |
|---|
| InChI: | InChI=1S/C10H12N5O6P/c11-8-5-9(13-2-12-8)15(3-14-5)10-7-6(4(1-16)19-10)20-22(17,18)21-7/h2-4,6-7,10,16H,1H2,(H,17,18)(H2,11,12,13)/p-1/t4-,6-,7-,10-/m1/s1 |
|---|
| CAS
number: |
634-01-5 |
|---|
| IUPAC Name: | adenosine 2',3'-phosphate |
|---|
|
Traditional IUPAC Name: |
4-(6-aminopurin-9-yl)-2-hydroxy-6-(hydroxymethyl)-tetrahydro-2???furo[3,4-d][1,3,2]dioxaphosphol-2-one |
|---|
| SMILES: | C4(N=C3(N(C1(C2(C(C(O1)CO)OP(O2)([O-])=O)))C=NC3=C(N=4)N)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as 2',3'-cyclic purine nucleotides. These are purine nucleotides in which the oxygen atoms linked to the C2 and C3 carbon atoms of the ribose moiety are both bonded the same phosphorus atom of the phosphate group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
2',3'-cyclic purine nucleotides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 2',3'-cyclic purine ribonucleotide
- Ribonucleoside 3'-phosphate
- 6-aminopurine
- Purine
- Imidazopyrimidine
- Aminopyrimidine
- Organic phosphoric acid derivative
- N-substituted imidazole
- Primary aromatic amine
- Pyrimidine
- Imidolactam
- Monosaccharide
- Azole
- Tetrahydrofuran
- 1,3_dioxaphospholane
- Imidazole
- Heteroaromatic compound
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Primary amine
- Alcohol
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Amine
- Primary alcohol
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Krall JF, Swensen JL, Korenman SG: Hormonal control of uterine contraction. Characterization od cyclic AMP-dependent membrane properties in the myometrium. Biochim Biophys Acta. 1976 Nov 2;448(4):578-88. [184841 ]
- Schoffstall AM: Prebiotic phosphorylation of nucleosides in formamide. Orig Life. 1976 Dec;7(4):399-412. [1023139 ]
- Cheng C, Fan C, Wan R, Tong C, Miao Z, Chen J, Zhao Y: Phosphorylation of adenosine with trimetaphosphate under simulated prebiotic conditions. Orig Life Evol Biosph. 2002 Jun;32(3):219-24. [12227426 ]
- Ghandour MS, Derer P, Labourdette G, Delaunoy JP, Langley OK: Glial cell markers in the reeler mutant mouse: a biochemical and immunohistological study. J Neurochem. 1981 Jan;36(1):195-200. [6257845 ]
- Wells MR, Sprinkle TJ: Purification of rat 2',3'-cyclic nucleotide 3'-phosphodiesterase. J Neurochem. 1981 Feb;36(2):633-9. [6257858 ]
- Reddy NB, Askanas V, Engel WK: Demonstration of 2',3'-cyclic nucleotide 3'-phosphohydrolase in cultured human Schwann cells. J Neurochem. 1982 Sep;39(3):887-9. [6284879 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|