|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120466 |
|---|
|
Identification |
|---|
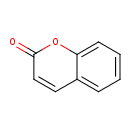
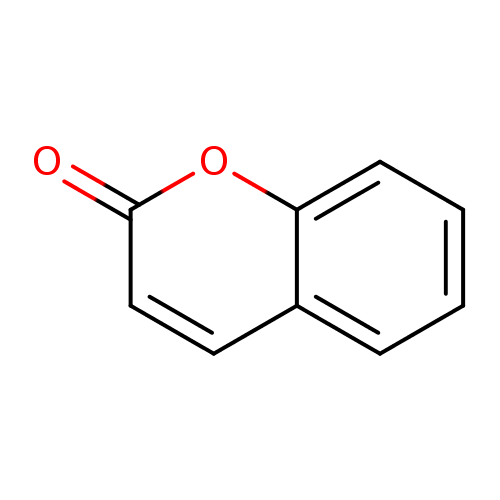
| Name: |
coumarin |
|---|
| Description: | A chromenone having the keto group located at the 2-position. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 1,2-Benzopyrone
- 2-Propenoic acid, 3-(2-hydroxyphenyl)-, d-lactone
- 2-Propenoic acid, 3-(2-hydroxyphenyl)-, delta-lactone
- 2H-1-Benzopyran-2-one
- 2H-benzo[b]pyran-2-one
- 5,6-Benzo-2-pyrone
- Benzo-a-pyrone
- Benzo-alpha-pyrone
- cis-o-Coumarinic acid lactone
- Coumarine
- Coumarine
- Coumarinic anhydride
- Cumarin
- o-hydroxycinnamic acid δ-lactone
- o-Hydroxycinnamic acid lactone
- Rattex
- Tonka bean camphor
|
|---|
|
Chemical Formula: |
C9H6O2 |
|---|
| Average Molecular Weight: |
146.145 |
|---|
| Monoisotopic Molecular
Weight: |
146.03677 |
|---|
| InChI Key: |
ZYGHJZDHTFUPRJ-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C9H6O2/c10-9-6-5-7-3-1-2-4-8(7)11-9/h1-6H |
|---|
| CAS
number: |
91-64-5 |
|---|
| IUPAC Name: | 2H-chromen-2-one |
|---|
|
Traditional IUPAC Name: |
coumarin |
|---|
| SMILES: | C1(OC2(=CC=CC=C(C=C1)2))=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as coumarins and derivatives. These are polycyclic aromatic compounds containing a 1-benzopyran moiety with a ketone group at the C2 carbon atom (1-benzopyran-2-one). |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Phenylpropanoids and polyketides |
|---|
| Sub Class | Coumarins and derivatives |
|---|
|
Direct Parent |
Coumarins and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Coumarin
- 1-benzopyran
- Benzopyran
- Pyranone
- Benzenoid
- Pyran
- Heteroaromatic compound
- Lactone
- Oxacycle
- Organoheterocyclic compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
71 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 71 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 1.9 mg/mL | Not Available | | LogP | 2.23 | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS | splash10-014v-6900000000-3e901733dc003512f338 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0002-0900000000-6ef9288c71771dd56774 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0006-9100000000-93154d9b40c4d0fdd1ae | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-014i-9000000000-72ec22913d1d574be75b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-7L) , Positive | splash10-00kb-8900000000-89e86d11a0dc2841af42 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-6M) , Positive | splash10-014i-7900000000-25bab36f91f71ac65817 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (JEOL JMS-AX-505-H) , Positive | splash10-02tj-9600000000-6e4062e14fc72534c96a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-e5519a8faf9232fc0fcc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-a6dc6c6c3032891bd134 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-4900000000-139cc9674e0b2836e441 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-e1154242f99d56bc5ed4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-d6df7c01bbecf91182c9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-1900000000-961e88f9b351b2d27bec | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-00kb-9700000000-7a649c1e257a7ad17b9d | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Vocanson M, Valeyrie M, Rozières A, Hennino A, Floc'h F, Gard A, Nicolas JF (2007)Lack of evidence for allergenic properties of coumarin in a fragrance allergy mouse model. Contact dermatitis 57, Pubmed: 17988284
- Abraham K, Pfister M, Wöhrlin F, Lampen A (2011)Relative bioavailability of coumarin from cinnamon and cinnamon-containing foods compared to isolated coumarin: a four-way crossover study in human volunteers. Molecular nutrition & food research 55, Pubmed: 21462332
- Marcolan M, Martins PA, Pedrosa VA, Rodrigues MR, de Oliveira HP, Codognoto L (2011)Spectrofluorimetric determination of coumarin in commercial tablets. Journal of fluorescence 21, Pubmed: 21046436
- Coltro WK, Lunte SM, Carrilho E (2008)Comparison of the analytical performance of electrophoresis microchannels fabricated in PDMS, glass, and polyester-toner. Electrophoresis 29, Pubmed: 19025869
- Weigt S, Huebler N, Strecker R, Braunbeck T, Broschard TH (2012)Developmental effects of coumarin and the anticoagulant coumarin derivative warfarin on zebrafish (Danio rerio) embryos. Reproductive toxicology (Elmsford, N.Y.) 33, Pubmed: 21798343
- Krasteva M, Peguet-Navarro J, Moulon C, Courtellemont P, Redziniak G, Schmitt D (1996)In vitro primary sensitization of hapten-specific T cells by cultured human epidermal Langerhans cells--a screening predictive assay for contact sensitizers. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 26, Pubmed: 8735869
|
|---|
| Synthesis Reference: |
Huang, Zhong-jing. Synthesis of coumarin under microwave irradiation. Guangxi Huagong (2001), 30(3), 1-2. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|