|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120449 |
|---|
|
Identification |
|---|
| Name: |
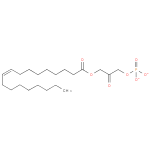
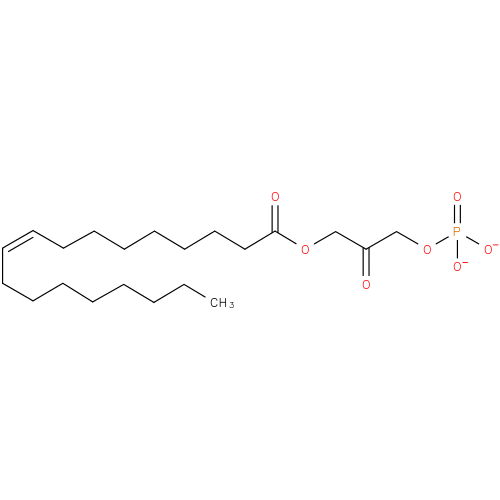
1-oleoyl-2-lyso-glycerone phosphate |
|---|
| Description: | A 1-acylglycerone 3-phosphate(2−) obtained by deprotonation of the phosphate OH groups of 1-oleoylglycerone 3-phosphate; major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 1-(9Z)-octadecenoyl-DHAP(2−)
- 1-(9Z)-octadecenoylglycerone 3-phosphate
- 1-(9Z)-octadecenoylglycerone 3-phosphate(2−)
|
|---|
|
Chemical Formula: |
C21H37O7P |
|---|
| Average Molecular Weight: |
432.493 |
|---|
| Monoisotopic Molecular
Weight: |
434.24335 |
|---|
| InChI Key: |
YZKFNNQAEBNCEN-KTKRTIGZSA-L |
|---|
| InChI: | InChI=1S/C21H39O7P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-21(23)27-18-20(22)19-28-29(24,25)26/h9-10H,2-8,11-19H2,1H3,(H2,24,25,26)/p-2/b10-9- |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 3-[(9Z)-octadec-9-enoyloxy]-2-oxopropyl phosphate |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CCCCCCCCC=CCCCCCCCC(=O)OCC(COP([O-])([O-])=O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as o-acylglycerone-phosphates. These are glycerone-3-phosphates carrying an acyl substituent at the 1-position. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
|
Class |
Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
|
Direct Parent |
O-acylglycerone-phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- O-acylglycerone-phosphate
- Monosaccharide phosphate
- Fatty acid ester
- Alpha-acyloxy ketone
- Monosaccharide
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Fatty acyl
- Alkyl phosphate
- Carboxylic acid ester
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organic oxide
- Organic anion
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- superpathway phosphatidate biosynthesis (yeast)PWY-7411
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|