|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120430 |
|---|
|
Identification |
|---|
| Name: |
vanillin |
|---|
| Description: | A member of the class of benzaldehydes carrying methoxy and hydroxy substituents at positions 3 and 4 respectively. |
|---|
|
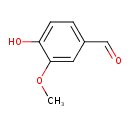
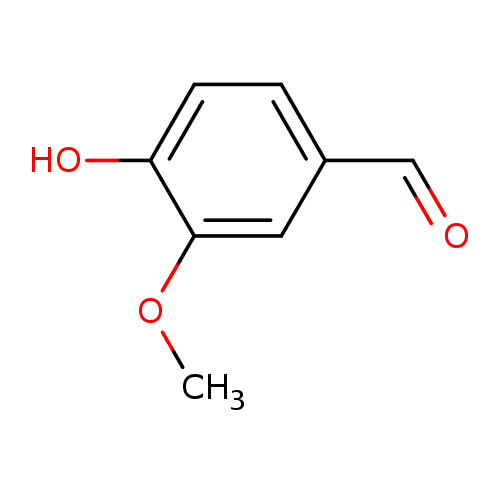
Structure |
|
|---|
| Synonyms: | - 3-methoxy-4-hydroxybenzaldehyde
- 4-formyl-2-methoxyphenol
- 4-hydroxy 3-methoxybenzaldehyde
- 4-Hydroxy-3-methoxy-benzaldehyde
- 4-hydroxy-3-methoxybenzaldehyde
- 4-Hydroxy-3-methoxybenzaldehyde
- 4-hydroxy-m-anisaldehyde
- methylprotocatechuic aldehyde
- p-hydroxy-m-methoxybenzaldehyde
- p-vanillin
- vaniline
- vanillaldehyde
- Vanillaldehyde
- vanillic aldehyde
- Vanillin
|
|---|
|
Chemical Formula: |
C8H8O3 |
|---|
| Average Molecular Weight: |
152.149 |
|---|
| Monoisotopic Molecular
Weight: |
152.04735 |
|---|
| InChI Key: |
MWOOGOJBHIARFG-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C8H8O3/c1-11-8-4-6(5-9)2-3-7(8)10/h2-5,10H,1H3 |
|---|
| CAS
number: |
121-33-5 |
|---|
| IUPAC Name: | 4-hydroxy-3-methoxybenzaldehyde |
|---|
|
Traditional IUPAC Name: |
vanillin |
|---|
| SMILES: | COC1(C(=CC=C(C=O)C=1)O) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as methoxyphenols. These are compounds containing a methoxy group attached to the benzene ring of a phenol moiety. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Benzenoids |
|---|
| Sub Class | Phenols |
|---|
|
Direct Parent |
Methoxyphenols |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Methoxyphenol
- Hydroxybenzaldehyde
- Anisole
- Benzaldehyde
- Benzoyl
- Phenoxy compound
- Phenol ether
- Methoxybenzene
- Alkyl aryl ether
- Aryl-aldehyde
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Ether
- Organooxygen compound
- Aldehyde
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
81.5 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 81.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 11 mg/mL at 25 °C | Not Available | | LogP | 1.21 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- vanillin and vanillate degradation IIPWY-7098
- vanillin and vanillate degradation IPWY-7097
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|