|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120425 |
|---|
|
Identification |
|---|
| Name: |
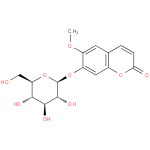
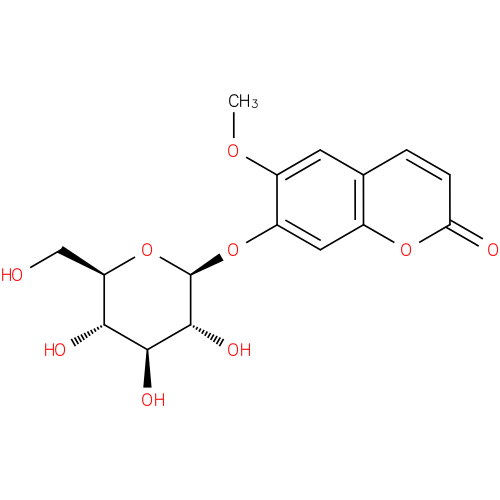
scopolin |
|---|
| Description: | A member of the class of coumarins that is scopoletin attached to a β-D-glucopyranosyl residue at position 7 via a glycosidic linkage. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 7-(beta-D-glucopyranosoyloxy)-6-methoxy-2H-1-benzopyran-2-one
- Scopolin
|
|---|
|
Chemical Formula: |
C16H18O9 |
|---|
| Average Molecular Weight: |
354.313 |
|---|
| Monoisotopic Molecular
Weight: |
354.0951 |
|---|
| InChI Key: |
SGTCGCCQZOUMJJ-YMILTQATSA-N |
|---|
| InChI: | InChI=1S/C16H18O9/c1-22-9-4-7-2-3-12(18)23-8(7)5-10(9)24-16-15(21)14(20)13(19)11(6-17)25-16/h2-5,11,13-17,19-21H,6H2,1H3/t11-,13-,14+,15-,16-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 6-methoxy-2-oxo-2H-chromen-7-yl β-D-glucopyranoside |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | COC2(=CC1(=C(OC(C=C1)=O)C=C2OC3(C(C(C(O)C(CO)O3)O)O))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as coumarin glycosides. These are aromatic compounds containing a carbohydrate moiety glycosidically bound to a coumarin moiety. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
|
Class |
Coumarins and derivatives |
|---|
| Sub Class | Coumarin glycosides |
|---|
|
Direct Parent |
Coumarin glycosides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Coumarin-7-o-glycoside
- Coumarin o-glycoside
- Phenolic glycoside
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Benzopyran
- 1-benzopyran
- Anisole
- Alkyl aryl ether
- Pyranone
- Benzenoid
- Monosaccharide
- Oxane
- Pyran
- Heteroaromatic compound
- Secondary alcohol
- Lactone
- Acetal
- Organoheterocyclic compound
- Ether
- Oxacycle
- Polyol
- Organooxygen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Primary alcohol
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
- a small molecule (SCOPOLIN)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Yuan T, Wan C, González-Sarrías A, Kandhi V, Cech NB, Seeram NP (2011)Phenolic glycosides from sugar maple (Acer saccharum) bark. Journal of natural products 74, Pubmed: 22032697
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|