|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120411 |
|---|
|
Identification |
|---|
| Name: |
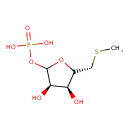
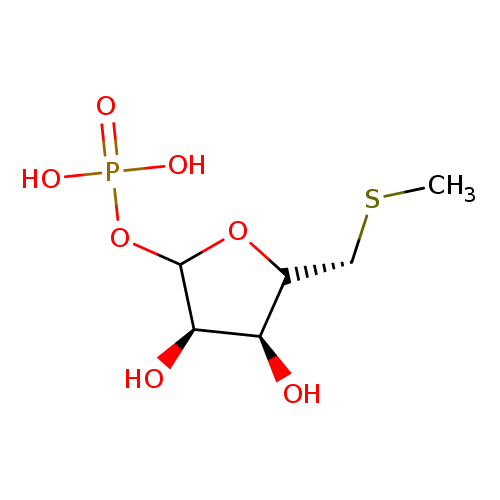
S-methyl-5-thio-α-D-ribose 1-phosphate |
|---|
| Description: | Dianion of S-methyl-5-thio-α-D-ribose 1-phosphate. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 5-methylthioribose-1-phosphate
- S5-methyl-5-thio-D-ribose-1-phosphate
- 5-methylthio-D-ribose-1-phosphate
- 5-MTR-1-P1-phosphomethylthioribose
- 1-phospho-5-S-methylthioribose
- 1-PMTR1-phospho-5-S-methylthio-α-D-ribofuranoside
- S-methyl-5-thio-α-D-ribose 1-phosphate
- S-methyl-5-thio-D-ribose 1-phosphate
|
|---|
|
Chemical Formula: |
C6H11O7PS |
|---|
| Average Molecular Weight: |
258.182 |
|---|
| Monoisotopic Molecular
Weight: |
260.01196 |
|---|
| InChI Key: |
JTFITTQBRJDSTL-JDJSBBGDSA-L |
|---|
| InChI: | InChI=1S/C6H13O7PS/c1-15-2-3-4(7)5(8)6(12-3)13-14(9,10)11/h3-8H,2H2,1H3,(H2,9,10,11)/p-2/t3-,4-,5-,6?/m1/s1 |
|---|
| CAS
number: |
72843-83-5 |
|---|
| IUPAC Name: | 5-S-methyl-1-O-phosphonato-5-thio-α-D-ribofuranose |
|---|
|
Traditional IUPAC Name: |
[(3R,4S)-3,4-dihydroxy-5-[(methylsulfanyl)methyl]oxolan-2-yl]oxyphosphonic acid |
|---|
| SMILES: | CSCC1(OC(OP([O-])(=O)[O-])C(C1O)O) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pentoses. These are monosaccharides in which the carbohydrate moiety contains five carbon atoms. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Pentoses |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pentose monosaccharide
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Oxolane
- 1,2-diol
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Dialkylthioether
- Sulfenyl compound
- Thioether
- Organic oxide
- Alcohol
- Hydrocarbon derivative
- Organosulfur compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Avila MA, Garcia-Trevijano ER, Lu SC, Corrales FJ, Mato JM: Methylthioadenosine. Int J Biochem Cell Biol. 2004 Nov;36(11):2125-30. [15313459 ]
- Savarese TM, Ghoda LY, Dexter DL, Parks RE Jr: Conversion of 5'-deoxy-5'-methylthioadenosine and 5'-deoxy-5'-methylthioinosine to methionine in cultured human leukemic cells. Cancer Res. 1983 Oct;43(10):4699-702. [6411330 ]
- Tisdale MJ: Methionine synthesis from 5'-methylthioadenosine by tumour cells. Biochem Pharmacol. 1983 Oct 1;32(19):2915-20. [6626263 ]
- Savarese TM, Cannistra AJ, Parks RE Jr, Secrist JA 3rd, Shortnacy AT, Montgomery JA: 5'-deoxy-5'-methylthioadenosine phosphorylase--IV. Biological activity of 2-fluoroadenine-substituted 5'-deoxy-5'-methylthioadenosine analogs. Biochem Pharmacol. 1987 Jun 15;36(12):1881-93. [3109431 ]
- Ghoda LY, Savarese TM, Dexter DL, Parks RE Jr, Trackman PC, Abeles RH: Characterization of a defect in the pathway for converting 5'-deoxy-5'-methylthioadenosine to methionine in a subline of a cultured heterogeneous human colon carcinoma. J Biol Chem. 1984 Jun 10;259(11):6715-9. [6725268 ]
- Gianotti AJ, Tower PA, Sheley JH, Conte PA, Spiro C, Ferro AJ, Fitchen JH, Riscoe MK: Selective killing of Klebsiella pneumoniae by 5-trifluoromethylthioribose. Chemotherapeutic exploitation of the enzyme 5-methylthioribose kinase. J Biol Chem. 1990 Jan 15;265(2):831-7. [2153115 ]
|
|---|
| Synthesis Reference: |
Della Ragione, Fulvio; Carteni-Farina, Maria; Gragnaniello, Vincenzo; Schettino, Maria Irene; Zappia, Vincenzo. Purification and characterization of 5'-deoxy-5'-methylthioadenosine phosphorylase from human placenta. Journal of Biological Chemistry (1986), 261(26), 12324-9. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|