|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120385 |
|---|
|
Identification |
|---|
| Name: |
2-hydroxyphenylacetate |
|---|
| Description: | The monocarboxylic acid anion formed from (2-hydroxyphenyl)acetic acid by loss of a proton from the carboxy group; major microspecies at pH 7.3. |
|---|
|
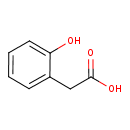
Structure |
|
|---|
| Synonyms: | - (2-hydroxyphenyl)acetate
- 2-hydroxybenzeneacetic acid (1−)
- 2-hydroxyphenylacetate
- o-hydroxyphenylacetate
|
|---|
|
Chemical Formula: |
C8H7O3 |
|---|
| Average Molecular Weight: |
151.141 |
|---|
| Monoisotopic Molecular
Weight: |
152.04735 |
|---|
| InChI Key: |
CCVYRRGZDBSHFU-UHFFFAOYSA-M |
|---|
| InChI: | InChI=1S/C8H8O3/c9-7-4-2-1-3-6(7)5-8(10)11/h1-4,9H,5H2,(H,10,11)/p-1 |
|---|
| CAS
number: |
614-75-5 |
|---|
| IUPAC Name: | (2-hydroxyphenyl)acetate |
|---|
|
Traditional IUPAC Name: |
o-hydroxyphenylacetic acid |
|---|
| SMILES: | C(=O)([O-])CC1(C=CC=CC(O)=1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as 2(hydroxyphenyl)acetic acids. These are phenylacetic acids that carry a hydroxyl group at the 2-position. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Benzenoids |
|---|
| Sub Class | Benzene and substituted derivatives |
|---|
|
Direct Parent |
2(hydroxyphenyl)acetic acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 2(hydroxyphenyl)acetic acid
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
145 - 147 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 145 - 147 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 44 mg/mL | Not Available | | LogP | 0.85 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Zelnícek E, Podhradská O, Sláma J (1970)[Phenylpyruvate and o-hydroxyphenylacetate in the urine of phenylketonurics treated by dietary measures] Zeitschrift fur Kinderheilkunde 108, Pubmed: 5475034
- Benavides J, Gimenez C, Valdivieso F, Mayor F (1976)Effect of phenylalanine metabolites on the activities of enzymes of ketone-body utilization in brain of suckling rats. The Biochemical journal 160, Pubmed: 12750
- Zelnícek E, Sláma J (1971)Phenylpyruvate and o-hydroxyphenylacetate in phenylketonuric urine. Clinica chimica acta; international journal of clinical chemistry 35, Pubmed: 5125336
|
|---|
| Synthesis Reference: |
Levine, Joseph; Eble, T. E.; Fischbach, Henry. Preparation of o-hydroxyphenylacetic acid. Journal of the American Chemical Society (1948), 70 1930. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|