|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120376 |
|---|
|
Identification |
|---|
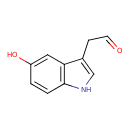
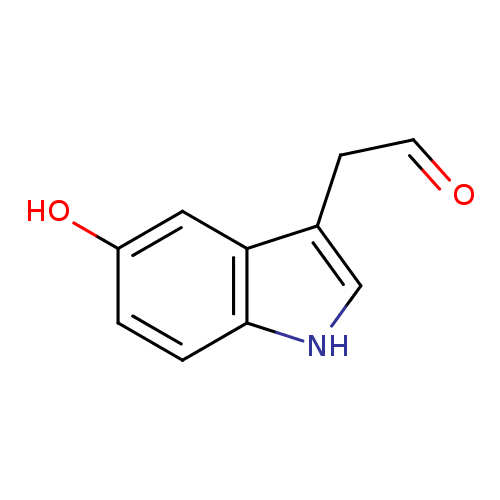
| Name: |
5-hydroxyindole acetaldehyde |
|---|
| Description: | An aldehyde that is acetaldehyde substituted by a 5-hydroxyindol-3-yl group. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (5-hydroxyindol-3-yl)acetaldehyde
- 5-Hial
- 5-Hydroxyindole-3-acetaldehyde
- 5-Hydroxyindoleacetaldehyde
|
|---|
|
Chemical Formula: |
C10H9NO2 |
|---|
| Average Molecular Weight: |
175.187 |
|---|
| Monoisotopic Molecular
Weight: |
175.06332 |
|---|
| InChI Key: |
OBFAPCIUSYHFIE-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C10H9NO2/c12-4-3-7-6-11-10-2-1-8(13)5-9(7)10/h1-2,4-6,11,13H,3H2 |
|---|
| CAS
number: |
1892-21-3 |
|---|
| IUPAC Name: | (5-hydroxy-1H-indol-3-yl)acetaldehyde |
|---|
|
Traditional IUPAC Name: |
hydroxyindoleacetaldehyde |
|---|
| SMILES: | C1(NC2(C(C(CC=O)=1)=CC(=CC=2)O)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as hydroxyindoles. These are organic compounds containing an indole moiety that carries a hydroxyl group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Indoles and derivatives |
|---|
|
Direct Parent |
Hydroxyindoles |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hydroxyindole
- 3-alkylindole
- Indole
- 1-hydroxy-2-unsubstituted benzenoid
- Substituted pyrrole
- Benzenoid
- Alpha-hydrogen aldehyde
- Pyrrole
- Heteroaromatic compound
- Azacycle
- Aldehyde
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Carbonyl group
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Some M, Svensson S, Hoog JO, Helander A: Studies on the interaction between ethanol and serotonin metabolism in rat, using deuterated ethanol and 4-methylpyrazole. Biochem Pharmacol. 2000 Feb 15;59(4):385-91. [10644046 ]
|
|---|
| Synthesis Reference: |
Marchand, B.; Streffer, Ch. Synthesis of 5-hydroxy-3-indolylacetaldehyde. Angew. Chem. (1959), 71 575. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|