|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120338 |
|---|
|
Identification |
|---|
| Name: |
1-O-methylsalicylate |
|---|
| Description: | A benzoate ester that is the methyl ester of salicylic acid. |
|---|
|
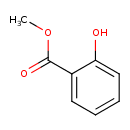
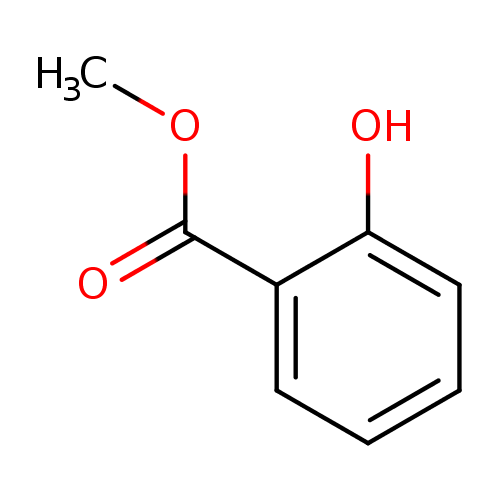
Structure |
|
|---|
| Synonyms: | - 2-(Methoxycarbonyl)phenol
- 2-Carbomethoxyphenol
- 2-Hydroxybenzoic acid methyl ester
- Betula oil
- Gaultheria oil
- Methyl 2-hydroxybenzoate
- Methyl o-hydroxybenzoate
- methyl salicylate
- Natural wintergreen oil
- Oil of wintergreen
- Spicewood Oil
- Sweet birch oil
- Teaberry oil
|
|---|
|
Chemical Formula: |
C8H8O3 |
|---|
| Average Molecular Weight: |
152.149 |
|---|
| Monoisotopic Molecular
Weight: |
152.04735 |
|---|
| InChI Key: |
OSWPMRLSEDHDFF-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C8H8O3/c1-11-8(10)6-4-2-3-5-7(6)9/h2-5,9H,1H3 |
|---|
| CAS
number: |
119-36-8 |
|---|
| IUPAC Name: | methyl 2-hydroxybenzoate |
|---|
|
Traditional IUPAC Name: |
methyl salicylate |
|---|
| SMILES: | COC(C1(C=CC=CC=1O))=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as o-hydroxybenzoic acid esters. These are benzoic acid esters where the benzene ring is ortho-substituted with a hydroxy group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Benzenoids |
|---|
| Sub Class | Benzene and substituted derivatives |
|---|
|
Direct Parent |
o-Hydroxybenzoic acid esters |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- O-hydroxybenzoic acid ester
- Salicylic acid or derivatives
- Benzoyl
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Vinylogous acid
- Methyl ester
- Carboxylic acid ester
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organic oxygen compound
- Organic oxide
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
-8.6 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | -8.6 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.7 mg/mL at 30 °C | Not Available | | LogP | 2.55 | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kappers IF, Hoogerbrugge H, Bouwmeester HJ, Dicke M (2011)Variation in herbivory-induced volatiles among cucumber (Cucumis sativus L.) varieties has consequences for the attraction of carnivorous natural enemies. Journal of chemical ecology 37, Pubmed: 21249432
- Ito K, Ito M (2011)Sedative effects of vapor inhalation of the essential oil of Microtoena patchoulii and its related compounds. Journal of natural medicines 65, Pubmed: 21287406
- Mallinger RE, Hogg DB, Gratton C (2011)Methyl salicylate attracts natural enemies and reduces populations of soybean aphids (Hemiptera: Aphididae) in soybean agroecosystems. Journal of economic entomology 104, Pubmed: 21404848
- Tamilvanan S, Karmegam S (2012)In vitro evaluation of chitosan coated- and uncoated-calcium alginate beads containing methyl salicylate-lactose physical mixture. Pharmaceutical development and technology 17, Pubmed: 21609308
- Yun LJ, Chen WL (2011)SA and ROS are involved in methyl salicylate-induced programmed cell death in Arabidopsis thaliana. Plant cell reports 30, Pubmed: 21327960
- Zhang X, Shen L, Li F, Meng D, Sheng J (2011)Methyl salicylate-induced arginine catabolism is associated with up-regulation of polyamine and nitric oxide levels and improves chilling tolerance in cherry tomato fruit. Journal of agricultural and food chemistry 59, Pubmed: 21790190
- Dekker T, Ignell R, Ghebru M, Glinwood R, Hopkins R (2011)Identification of mosquito repellent odours from Ocimum forskolei. Parasites & vectors 4, Pubmed: 21936953
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|