|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120336 |
|---|
|
Identification |
|---|
| Name: |
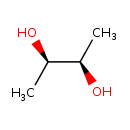
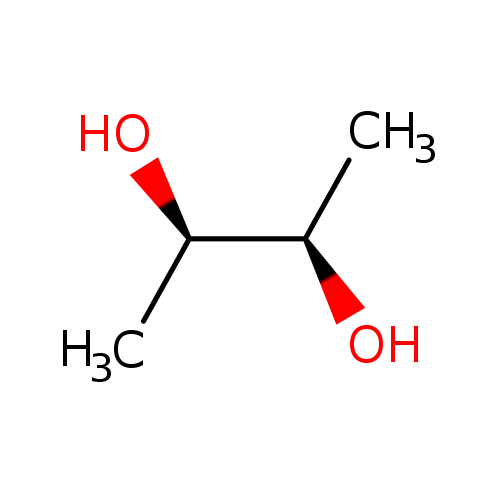
(R,R)-2,3-butanediol |
|---|
| Description: | The (R,R) diastereoisomer of butane-2,3-diol. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (R,R)-(−)-butane-2,3-diol
- (R,R)-2,3-Butanediol
- (R,R)-2,3-BUTANEDIOL
- (R,R)-2,3-Butylene glycol
- (R,R)-Butane-2,3-diol
- (R,R)-butane-2,3-diol
|
|---|
|
Chemical Formula: |
C4H10O2 |
|---|
| Average Molecular Weight: |
90.122 |
|---|
| Monoisotopic Molecular
Weight: |
90.06808 |
|---|
| InChI Key: |
OWBTYPJTUOEWEK-QWWZWVQMSA-N |
|---|
| InChI: | InChI=1S/C4H10O2/c1-3(5)4(2)6/h3-6H,1-2H3/t3-,4-/m1/s1 |
|---|
| CAS
number: |
24347-58-8 |
|---|
| IUPAC Name: | (2R,3R)-butane-2,3-diol |
|---|
|
Traditional IUPAC Name: |
(R,R)-butane-2,3-diol |
|---|
| SMILES: | CC(C(O)C)O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as 1,2-diols. These are polyols containing an alcohol group at two adjacent positions. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organooxygen compounds |
|---|
|
Class |
Alcohols and polyols |
|---|
| Sub Class | Polyols |
|---|
|
Direct Parent |
1,2-diols |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Secondary alcohol
- 1,2-diol
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
19.7 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 19.7 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 0.88 | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|