|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120331 |
|---|
|
Identification |
|---|
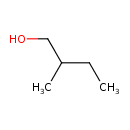
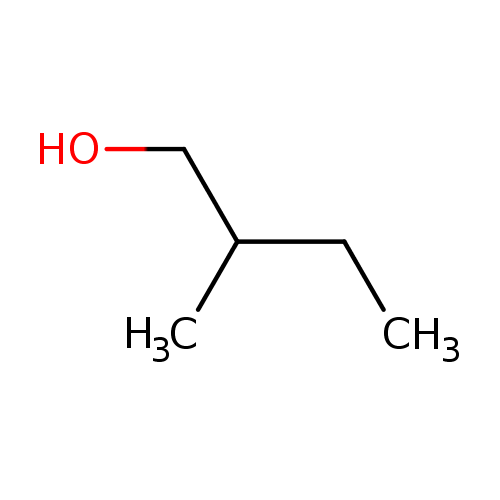
| Name: |
2-methylbutanol |
|---|
| Description: | A primary alcohol that is isopentane substituted by a hydroxy group at position 1. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2-methyl butanol-1
- 2-methyl-1-butanol
- 2-methyl-n-butanol
- 2-methylbutanol
- 2-methylbutyl alcohol
- active amyl alcohol
- active primary amyl alcohol
- CH3CH2CH(CH3)CH2OH
- methyl-2-butan-1-ol
- primary active amyl alcohol
- sec-butylcarbinol
|
|---|
|
Chemical Formula: |
C5H12O |
|---|
| Average Molecular Weight: |
88.149 |
|---|
| Monoisotopic Molecular
Weight: |
88.08881 |
|---|
| InChI Key: |
QPRQEDXDYOZYLA-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C5H12O/c1-3-5(2)4-6/h5-6H,3-4H2,1-2H3 |
|---|
| CAS
number: |
1565-80-6 |
|---|
| IUPAC Name: | 2-methylbutan-1-ol |
|---|
|
Traditional IUPAC Name: |
2-methyl-1-butanol |
|---|
| SMILES: | CCC(CO)C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as primary alcohols. These are compounds comprising the primary alcohol functional group, with the general structure RCOH (R=alkyl, aryl). |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Primary alcohols |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hydrocarbon derivative
- Primary alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0079-9000000000-128169279e6dbc93647d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9000000000-dc20fc6a9edf20c015c0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ab9-9000000000-c22c4d3aed422eaa3d6c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0079-9000000000-128169279e6dbc93647d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9000000000-dc20fc6a9edf20c015c0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ab9-9000000000-c22c4d3aed422eaa3d6c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9000000000-3755227e402feb51b4de | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9000000000-fcef5652b712c7416e06 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-52f93c4b7493a853c052 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9000000000-3755227e402feb51b4de | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9000000000-fcef5652b712c7416e06 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-52f93c4b7493a853c052 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-0a4i-9000000000-354c4fb764d5125305e7 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Baena-Ruano S, Santos-Dueñas IM, Mauricio JC, García-García I (2010)Relationship between changes in the total concentration of acetic acid bacteria and major volatile compounds during the acetic acid fermentation of white wine. Journal of the science of food and agriculture 90, Pubmed: 20812374
- Amaro AL, Fundo JF, Oliveira A, Beaulieu JC, Fernández-Trujillo JP, Almeida DP (2013)1-methylcyclopropene effects on temporal changes of aroma volatiles and phytochemicals of fresh-cut cantaloupe. Journal of the science of food and agriculture 93, Pubmed: 22821412
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|