|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120311 |
|---|
|
Identification |
|---|
| Name: |
dGMP |
|---|
| Description: | A 2'-deoxyribonucleoside 5'-monophosphate(2−) obtained by deprotonation of the phosphate OH groups of 2'-deoxyguanosine 5'-monophosphate (dGMP). |
|---|
|
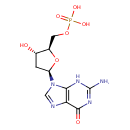
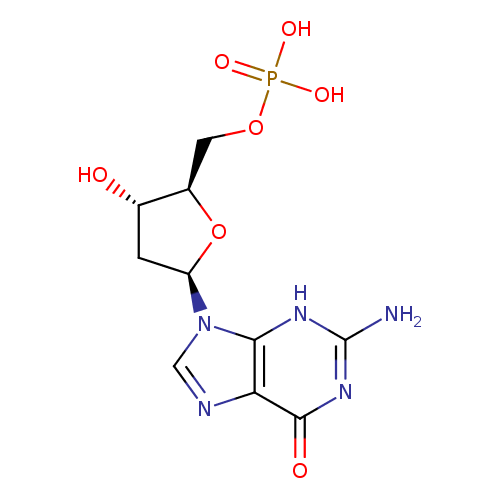
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C10H12N5O7P |
|---|
| Average Molecular Weight: |
345.208 |
|---|
| Monoisotopic Molecular
Weight: |
347.06308 |
|---|
| InChI Key: |
LTFMZDNNPPEQNG-KVQBGUIXSA-L |
|---|
| InChI: | InChI=1S/C10H14N5O7P/c11-10-13-8-7(9(17)14-10)12-3-15(8)6-1-4(16)5(22-6)2-21-23(18,19)20/h3-6,16H,1-2H2,(H2,18,19,20)(H3,11,13,14,17)/p-2/t4-,5+,6+/m0/s1 |
|---|
| CAS
number: |
902-04-5 |
|---|
| IUPAC Name: | 2'-deoxy-5'-O-phosphonatoguanosine |
|---|
|
Traditional IUPAC Name: |
deoxyguanylate |
|---|
| SMILES: | C(OP(=O)([O-])[O-])C1(OC(CC(O)1)N3(C=NC2(C(=O)NC(N)=NC=23))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as purine 2'-deoxyribonucleoside monophosphates. These are purine nucleotides with monophosphate group linked to the ribose moiety lacking a hydroxyl group at position 2. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
Purine 2'-deoxyribonucleoside monophosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine 2'-deoxyribonucleoside monophosphate
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Hydroxypyrimidine
- N-substituted imidazole
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Alkyl phosphate
- Heteroaromatic compound
- Imidazole
- Azole
- Oxolane
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Organic oxygen compound
- Organopnictogen compound
- Organic nitrogen compound
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0fr2-0598000000-21b063e2aff7146d8568 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0udi-0900000000-d2858e957ac8a4c91e79 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-004j-9203000000-40bcf8d5e1ae6813c4f7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Warnecke A, Fichtner I, Garmann D, Jaehde U, Kratz F: Synthesis and biological activity of water-soluble maleimide derivatives of the anticancer drug carboplatin designed as albumin-binding prodrugs. Bioconjug Chem. 2004 Nov-Dec;15(6):1349-59. [15546202 ]
|
|---|
| Synthesis Reference: |
Reichard, Peter. Formation of deoxyguanosine 5'-phosphate from guanosine 5'-phosphate with enzymes from chick embryos. Biochimica et Biophysica Acta (1960), 41 368-9. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|