|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120287 |
|---|
|
Identification |
|---|
| Name: |
dGDP |
|---|
| Description: | A 2'-deoxyribonucleoside 5'-diphosphate obtained by deprotonation of the diphosphate OH groups of dGDP. |
|---|
|
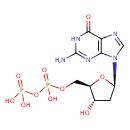
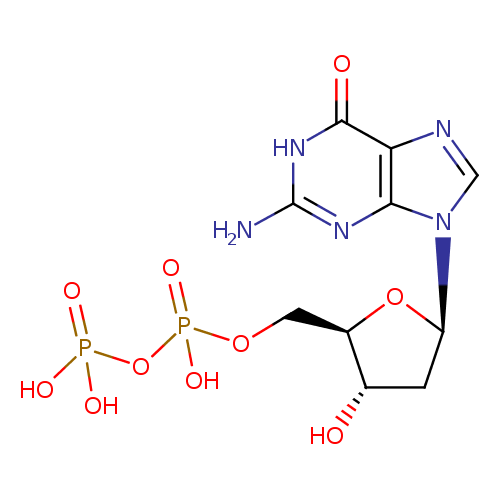
Structure |
|
|---|
| Synonyms: | - 2'-deoxyguanosine 5'-diphosphate
- dGDP
|
|---|
|
Chemical Formula: |
C10H12N5O10P2 |
|---|
| Average Molecular Weight: |
424.18 |
|---|
| Monoisotopic Molecular
Weight: |
427.02942 |
|---|
| InChI Key: |
CIKGWCTVFSRMJU-KVQBGUIXSA-K |
|---|
| InChI: | InChI=1S/C10H15N5O10P2/c11-10-13-8-7(9(17)14-10)12-3-15(8)6-1-4(16)5(24-6)2-23-27(21,22)25-26(18,19)20/h3-6,16H,1-2H2,(H,21,22)(H2,18,19,20)(H3,11,13,14,17)/p-3/t4-,5+,6+/m0/s1 |
|---|
| CAS
number: |
3493-09-2 |
|---|
| IUPAC Name: | [({[(2R,3S,5R)-5-(2-amino-6-oxo-6,9-dihydro-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]phosphonic acid |
|---|
|
Traditional IUPAC Name: |
dGDP |
|---|
| SMILES: | C(OP(=O)([O-])OP(=O)([O-])[O-])C1(OC(CC(O)1)N3(C=NC2(C(=O)NC(N)=NC=23))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as purine 2'-deoxyribonucleoside diphosphates. These are purine nucleotides with diphosphate group linked to the ribose moiety lacking a hydroxyl group at position 2. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
Purine 2'-deoxyribonucleoside diphosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine 2'-deoxyribonucleoside diphosphate
- 6-oxopurine
- Hypoxanthine
- Organic pyrophosphate
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Pyrimidone
- Monoalkyl phosphate
- N-substituted imidazole
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Primary aromatic amine
- Pyrimidine
- Alkyl phosphate
- Heteroaromatic compound
- Vinylogous amide
- Azole
- Oxolane
- Imidazole
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Azacycle
- Organic oxygen compound
- Alcohol
- Organic nitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Organopnictogen compound
- Organonitrogen compound
- Organooxygen compound
- Amine
- Primary amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 5.5 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Brady WA, Kokoris MS, Fitzgibbon M, Black ME: Cloning, characterization, and modeling of mouse and human guanylate kinases. J Biol Chem. 1996 Jul 12;271(28):16734-40. [8663313 ]
- Nomanbhoy TK, Leonard DA, Manor D, Cerione RA: Investigation of the GTP-binding/GTPase cycle of Cdc42Hs using extrinsic reporter group fluorescence. Biochemistry. 1996 Apr 9;35(14):4602-8. [8605211 ]
- Rothwell PS: The new MFGDP (UK) examination (formerly, the DGDP [UK]) Br Dent J. 1999 Oct 9;187(7):354-6. [10581811 ]
- Bialkowski K, Kasprzak KS: Inhibition of 8-oxo-2'-deoxyguanosine 5'-triphosphate pyrophosphohydrolase (8-oxo-dGTPase) activity of the antimutagenic human MTH1 protein by nucleoside 5'-diphosphates. Free Radic Biol Med. 2003 Sep 15;35(6):595-602. [12957652 ]
- Kumar V, Spangenberg O, Konrad M: Cloning of the guanylate kinase homologues AGK-1 and AGK-2 from Arabidopsis thaliana and characterization of AGK-1. Eur J Biochem. 2000 Jan;267(2):606-15. [10632732 ]
|
|---|
| Synthesis Reference: |
Klenow, Hans; Lichtler, Eleanor; Andersen, Bjorn. Adenine deoxyriboside polyphosphates. Acta Chemica Scandinavica (1956), 10 159-60 |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|