|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120275 |
|---|
|
Identification |
|---|
| Name: |
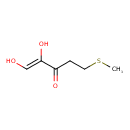
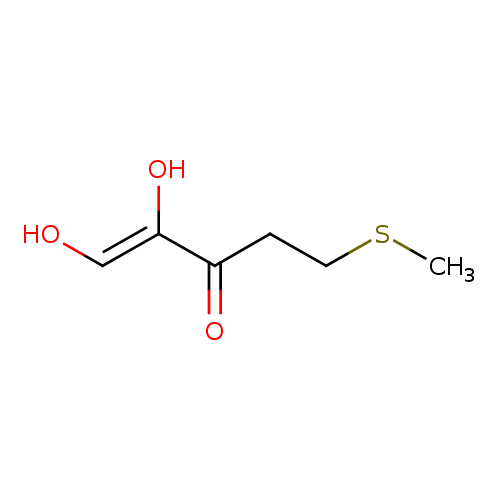
1,2-dihydroxy-5-(methylthio)pent-1-en-3-one |
|---|
| Description: | Conjugate base of 1,2-dihydroxy-5-(methylthio)pent-1-en-3-one arising from deprotonation of the 2-hydroxy group. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 1,2-dihydroxy-3-keto-5-methylthiopentene anion
- 1,2-dihydroxy-3-keto-5-methylthiopentane
- 1,2-dihydroxy-3-keto-5-methylthiopenteneacireductone
|
|---|
|
Chemical Formula: |
C6H9O3S |
|---|
| Average Molecular Weight: |
161.195 |
|---|
| Monoisotopic Molecular
Weight: |
162.03506 |
|---|
| InChI Key: |
CILXJJLQPTUUSS-XQRVVYSFSA-M |
|---|
| InChI: | InChI=1S/C6H10O3S/c1-10-3-2-5(8)6(9)4-7/h4,7,9H,2-3H2,1H3/p-1/b6-4- |
|---|
| CAS
number: |
746507-19-7 |
|---|
| IUPAC Name: | 1-hydroxy-5-(methylsulfanyl)-3-oxopent-1-en-2-olate |
|---|
|
Traditional IUPAC Name: |
(1Z)-1,2-dihydroxy-5-(methylsulfanyl)pent-1-en-3-one |
|---|
| SMILES: | CSCCC(C([O-])=CO)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as alpha-branched alpha,beta-unsaturated ketones. These are alpha,beta-unsaturated ketones that carry a branch on the alpha carbon. They have the generic structure RC(=O)C(R')=C, R = organyl group and R'= any heteroatom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Alpha-branched alpha,beta-unsaturated ketones |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-branched alpha,beta-unsaturated-ketone
- Vinylogous acid
- Enone
- Alpha-hydroxy ketone
- Acryloyl-group
- Ketone
- Enediol
- Dialkylthioether
- Sulfenyl compound
- Thioether
- Organic oxide
- Hydrocarbon derivative
- Organosulfur compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- S-methyl-5-thio-α-D-ribose 1-phosphate degradation IPWY-6755
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|